- published: 16 Feb 2013
- views: 25726
-
remove the playlistOzone Depletion
- remove the playlistOzone Depletion
- published: 27 Sep 2008
- views: 97142
- published: 31 Aug 2015
- views: 613
- published: 16 Apr 2014
- views: 14464
- published: 15 Sep 2011
- views: 82652
- published: 06 Nov 2012
- views: 101183
- published: 15 Jun 2015
- views: 4593
- published: 01 Jun 2013
- views: 115893
- published: 08 Feb 2013
- views: 21689

Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere (the ozone layer), and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon is referred to as the ozone hole. In addition to these well-known stratospheric phenomena, there are also springtime polar tropospheric ozone depletion events.
The details of polar ozone hole formation differ from that of mid-latitude thinning, but the most important process in both is catalytic destruction of ozone by atomic halogens. The main source of these halogen atoms in the stratosphere is photodissociation of man-made halocarbon refrigerants (CFCs, freons, halons). These compounds are transported into the stratosphere after being emitted at the surface. Both types of ozone depletion were observed to increase as emissions of halo-carbons increased.
CFCs and other contributory substances are referred to as ozone-depleting substances (ODS). Since the ozone layer prevents most harmful UVB wavelengths (280–315 nm) of ultraviolet light (UV light) from passing through the Earth's atmosphere, observed and projected decreases in ozone have generated worldwide concern leading to adoption of the Montreal Protocol that bans the production of CFCs, halons, and other ozone-depleting chemicals such as carbon tetrachloride and trichloroethane. It is suspected that a variety of biological consequences such as increases in skin cancer, cataracts, damage to plants, and reduction of plankton populations in the ocean's photic zone may result from the increased UV exposure due to ozone depletion.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 2:45
2:45Ozone Depletion
Ozone DepletionOzone Depletion
This video explains Ozone depletion in the Stratosphere, caused by the pollution of man-made compounds in addition to the lack of stratospheric ozone in Antarctica. -
 4:06
4:06OZONE DEPLETION
OZONE DEPLETIONOZONE DEPLETION
The phenomenon of ozone depletion and its links to possible hazard to life forms has captured international attention. India is a signatory to the Montreal Protocol, which sets the agenda for controlling ozone depletion by phasing out production and use of Chlorofluorocarbons. -
 3:50
3:50ozone depletion
ozone depletionozone depletion
Documentary made by my students of Health Safety and Environment engg. (University of petroleum and energy studies , Dehradun ) on Ozone Layer depletion for the initiative taken up by GREEN UP for bringing awareness . -
 1:32
1:32Ozone Depletion
Ozone DepletionOzone Depletion
brittany yang katherine kwon dharmesh patel -
![Ozone Layer and it's Depletion [Animation]; updated 16 Apr 2014; published 16 Apr 2014](http://web.archive.org./web/20160418091232im_/http://i.ytimg.com/vi/OMtZC4FV_zM/0.jpg) 1:23
1:23Ozone Layer and it's Depletion [Animation]
Ozone Layer and it's Depletion [Animation]Ozone Layer and it's Depletion [Animation]
See an organised list of all the animations: http://doctorprodigious.wordpress.com/hd-animations/ -
 16:37
16:37The Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific Journey
The Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific JourneyThe Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific Journey
While the ozone hole has been considered by some as a solved problem, in fact its recovery is still many decades away and the effects and interactions of ozone depletion on climate change are just starting to be understood. On the 16th September 2011, embark on an investigative journey through the history and science of the ozone layer, the actions taken to address this major environmental threat and the consequences both for the ozone layer and climate system. This short film seeks out explanations and answers from the scientists closest to the issue. -
 3:00
3:00What is Ozone Layer? | Mocomi Kids
What is Ozone Layer? | Mocomi KidsWhat is Ozone Layer? | Mocomi Kids
http://mocomi.com/ presents: What is Ozone layer? Life on earth is protected from the UV rays by a layer in the upper atmosphere (known as the stratosphere), which surrounds earth. This layer is called the Ozone layer. Ozone is a gas made up of three oxygen atoms (O3) much like the layer of butter that settles on top if a glass of buttermilk is left unattended for a while. This layer is just about 3-5mm thick. This thinly spread-out gas has been protecting life near the earth’s surface from the sun’s harmful UV rays for billions of years. Ozone is spread thinly throughout the stratosphere in low quantities. Watch this animated video to understand Why is the ozone layer in danger? and What is the ozone hole? To learn more about the ozone layer, go to http://mocomi.com/what-is-the-ozone-layer/ For more such interesting videos and interactive articles related to environment, visit: http://mocomi.com/learn/environment/ Follow Mocomi Kids, on Facebook https://www.facebook.com/mocomikids/ on Twitter https://twitter.com/MocomiKids on Pinterest https://www.pinterest.com/mocomikids/ on Google+ https://plus.google.com/+mocomikids/ on LinkedIn https://www.linkedin.com/company/mocomi-kids -
 4:25
4:25AIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENT
AIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENTAIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENT
-
 30:30
30:30Ozone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi Agrahari
Ozone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi AgrahariOzone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi Agrahari
Hope you enjoyed this video on Ozone Depletion by SuperProf Ravi Agrahari To watch more videos go to - http://bit.ly/1RTmgJf About the subject: Environment covers every living and non-living thing occurring naturally on Earth. Ecology is the scientific study of interactions between organisms and their environment. There are several practical applications of ecology in natural resource management, community health, economics, conservation biology and wetland management, among others. Biodiversity deals with different types of life found on earth. On Earth, biodiversity is not distributed evenly, rather it is the richest in the tropics. Studying Environment, Ecology and Bio-diversity is important for UPSC / IAS / IPS / IFS exam. About the Professor: Dr. Ravi P. Agrahari, Ph.D, IIT Delhi, New Delhi, India. Mr. Agrahari was born on December 2, 1981 at Gorakhpur UP in India. Mr. Agrahari completed his Masters in Botany in 2002 from the Deen Dayal Upadhyay Gorakhpur University, UP, India. About SuperProfs: SuperProfs is India's largest online coaching platform for competitive exam preparation. We help you prepare for CA, CS, CMA, UPSC, GATE and JEE, among other exams. Currently we have over 1 lakh registered students who are enjoying our 1000+ free video lecture courses. We have tied up with 200+ top professors from across the country. Our mobile app allows students to watch video lectures without any interruptions even with 2G data connections. In addition, students can also download video lectures on their mobiles and watch them offline - anywhere, anytime. Here is a quick preview of what's inside the SuperProfs app for you: You can install the SuperProfs app on your phone and tablet (for FREE) and watch video lectures wherever you go. You can now download CA / UPSC and other course video lectures onto your mobile phones and watch them offline. You can now play, pause, replay and rewind the video lectures any number of times you want. You can now access exam tips, daily news updates, supplementary study material and blog posts on our app. Our app automatically optimizes the video lecture streaming quality to accommodate both landscape and portrait mode of viewing. Join us and begin your coaching for CA / UPSC and other exams! For more information, call us on our toll-free number 1800-313-2122. -
 4:18
4:18Ozone Layer Danger
Ozone Layer DangerOzone Layer Danger
Think of the ozone layer as Earth's sunglasses, protecting life on the surface from the harmful glare of the sun's strongest ultraviolet rays, which can cause skin cancer and other maladies. Ozone stinks. People who breathe it gag as their lungs burn. The EPA classifies ground-level ozone as air pollution. Yet without it, life on Earth would be impossible. A fragile layer of ozone 25 km above Earth's surface is all that stands between us and some of the harshest UV rays from the sun. The ozone molecule O3 blocks radiation which would otherwise burn skin and cause cancer. On Mars, which has no ozone layer to protect it, solar UV rays strafe the surface with deadly effect, leaving the apparently lifeless planet without the simplest of organic molecules in the upper millimeters of exposed Martian soil. To keep track of our planet's ozone layer, NASA is about to launch the most sophisticated space-based ozone sensor ever: SAGE III, slated for installation on the International Space Station in 2014. People were understandably alarmed, then, in the 1980s when scientists noticed that manmade chemicals in the atmosphere were destroying this layer. Governments quickly enacted an international treaty, called the Montreal Protocol, to ban ozone-destroying gases such as CFCs then found in aerosol cans and air conditioners. -
 5:44
5:44How CFC's Deplete the Ozone Layer
How CFC's Deplete the Ozone Layer -
 2:02
2:02What happened to the holes in the ozone layer? | Global Ideas
What happened to the holes in the ozone layer? | Global IdeasWhat happened to the holes in the ozone layer? | Global Ideas
No sunbathing without sunscreen - that was a mantra for sun worshippers back in the 1980s. The reason: The sun's dangerous UV-radiation passes through massive holes in the Earth's ozone layer and strikes the Earth surface - and our skins - nearly unhindered. Today, we are still busy drenching ourselves in suncream, but it's got quiet about ozone holes. Just what happened to them? More information on: http://www.ideasforacoolerworld.org -
 2:56
2:56Causes of Ozone Depletion
Causes of Ozone DepletionCauses of Ozone Depletion
Useful for CBSE, ICSE, NCERT & International Students Grade : 10 Subject : Biology Lesson :Our environment Topic: Causes of Ozone Depletion Ozone layer depletion, is simply the wearing out (reduction) of the amount of ozone in the stratosphere. Unlike pollution, which has many types and causes, Ozone depletion has been pinned down to one major human activity. Visit www.oztern.com to find personalized test preparation solutions for Pre Medical - AIPMT, AIIMS, JIPMER, State, Pre Engineering - IIT JEE, JEE MAIN, BITSAT, State and Foundations - Class 6 to 10. -
 5:06
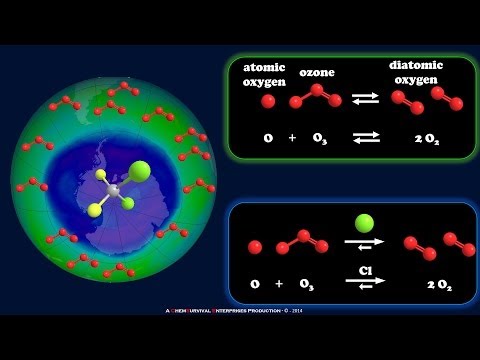
5:06Ozone Depletion - Role of Halocarbons
Ozone Depletion - Role of HalocarbonsOzone Depletion - Role of Halocarbons
Here's how halocarbons chew through ozone. Cl atoms react with an ozone molecule, but then the bare Cl atom is regenerated!
- Aerosol spray
- Air conditioning
- Air pollution
- Air quality
- Aircraft
- Albedo
- Allotropes
- Anne M. Burford
- Anoxic event
- Antarctic
- Antarctica
- Anthropogenic
- Arctic
- Arctic haze
- Argentina
- Asthma inhaler
- Atmospheric window
- Aura (satellite)
- Australia
- Bali Road Map
- Basal cell carcinoma
- Bibcode
- Biofuel
- Bioremediation
- Black carbon
- Bond event
- Brian G. Gardiner
- Bromine
- CALIPSO
- Carbon credit
- Carbon dioxide
- Carbon offset
- Carbon sink
- Carbon tax
- Carbon tetrachloride
- Catalysis
- Catalyst
- Catalytic
- Cataracts
- Chile
- Chlorine
- Chlorine nitrate
- Chlorofluorocarbon
- CLaMS
- Climate change
- Climate model
- Climate oscillation
- Climate sensitivity
- Cloud forcing
- Combustion
- Cosmic ray
- Cyanobacteria
- Dam
- Desalination
- Dimer (chemistry)
- Dobson unit
- Drought
- Drought tolerance
- DuPont
- Earth
- Earth's atmosphere
- Efficient energy use
- Emissions trading
- Environment Agency
- Environmental ethics
- Eutrophication
- Exponential decay
- Fertilizers
- Fission product
- Forest dieback
- Fossil fuel
- Free radical
- Freon
- G.M.B. Dobson
- Gaia hypothesis
- Geoengineering
- Glacial lake
- Glacial period
- Global climate model
- Global cooling
- Global dimming
- Global distillation
- Global warming
- Greenhouse effect
- Greenhouse gas
- Greenpeace
- Haloalkane
- Haloalkanes
- Halocarbon
- Halogen
- Halogens
- Halon
- Harvard University
- HCFC
- Herbicide
- Hindu Kush
- Hydrofluoric acid
- Hydrofluorocarbon
- Hydrogen chloride
- Hydroxyl radical
- Indian Ocean Dipole
- Indoor air quality
- Infrared window
- Iris hypothesis
- Irrigation
- James Hansen
- James Lovelock
- Joe Farman
- John Wiley & Sons
- Joint Implementation
- Jon Shanklin
- JSTOR
- Kyoto Protocol
- Land pollution
- Light pollution
- Low-carbon economy
- Marine debris
- Marine pollution
- Mario J. Molina
- Melanoma
- Methyl bromide
- Milankovitch cycles
- Montreal Protocol
- Mount Erebus
- NASA
- Natural environment
- Nature (journal)
- New Zealand
- Nitric oxide
- Nitrogen
- Nitrous oxide
- Noise pollution
- Nuclear fallout
- Nuclear power
- Null cycle
- Ocean acidification
- Oil spill
- Orbital forcing
- Organic compounds
- Oxygen
- Ozone
- Ozone depletion
- Ozone layer
- Ozone-oxygen cycle
- Paleoclimatology
- Paleotempestology
- Pantheon Books
- Particulates
- Paul Crutzen
- Pesticide
- Photic zone
- Photochemistry
- Photodissociation
- Phytoremediation
- Plankton
- Polar vortex
- Pollution
- Portal Ecology
- Portal Environment
- Proxy (climate)
- PubMed Central
- PubMed Identifier
- Punta Arenas
- Qinghai
- R-134a
- Radiation poisoning
- Radiative forcing
- Radiative transfer
- Rainwater tank
- Ralph Cicerone
- Redox
- Regime shift
- Renewable energy
- Rice
- Richard E. Benedick
- Robert Abplanalp
- Salt spray
- Scandinavia
- Season creep
- SGI Origin 2000
- Simple living
- Skin cancer
- SLIMCAT
- Smog
- Software
- Soil contamination
- Solar variation
- South Africa
- South pole
- Square kilometer
- Steven Wofsy
- Stratosphere
- Supersonic
- Surface runoff
- Susan Solomon
- Svante Arrhenius
- Template Pollution
- Thermal pollution
- Thomas Midgley, Jr.
- Tibet
- Troposphere
- Tropospheric
- Tropospheric ozone
- Turbosphere
- UC Irvine
- Ultraviolet
- Ultraviolet B
- UNEP
- Urban heat island
- Urban runoff
- UV Index
- UVB
- Visual pollution
- Vitamin D
- Volcanism
- Waste water
- Water pollution
- Water quality
- Water stagnation
- Waterborne diseases
- Watt
- Weather control
- WHO
- Wikipedia Link rot
- William Ruckelshaus
- Xinjiang
-

Ozone Depletion
This video explains Ozone depletion in the Stratosphere, caused by the pollution of man-made compounds in addition to the lack of stratospheric ozone in Antarctica. -

OZONE DEPLETION
The phenomenon of ozone depletion and its links to possible hazard to life forms has captured international attention. India is a signatory to the Montreal Protocol, which sets the agenda for controlling ozone depletion by phasing out production and use of Chlorofluorocarbons. -

ozone depletion
Documentary made by my students of Health Safety and Environment engg. (University of petroleum and energy studies , Dehradun ) on Ozone Layer depletion for the initiative taken up by GREEN UP for bringing awareness . -

Ozone Depletion
brittany yang katherine kwon dharmesh patel -

Ozone Layer and it's Depletion [Animation]
See an organised list of all the animations: http://doctorprodigious.wordpress.com/hd-animations/ -

The Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific Journey
While the ozone hole has been considered by some as a solved problem, in fact its recovery is still many decades away and the effects and interactions of ozone depletion on climate change are just starting to be understood. On the 16th September 2011, embark on an investigative journey through the history and science of the ozone layer, the actions taken to address this major environmental threat and the consequences both for the ozone layer and climate system. This short film seeks out explanations and answers from the scientists closest to the issue. -

What is Ozone Layer? | Mocomi Kids
http://mocomi.com/ presents: What is Ozone layer? Life on earth is protected from the UV rays by a layer in the upper atmosphere (known as the stratosphere), which surrounds earth. This layer is called the Ozone layer. Ozone is a gas made up of three oxygen atoms (O3) much like the layer of butter that settles on top if a glass of buttermilk is left unattended for a while. This layer is just about 3-5mm thick. This thinly spread-out gas has been protecting life near the earth’s surface from the sun’s harmful UV rays for billions of years. Ozone is spread thinly throughout the stratosphere in low quantities. Watch this animated video to understand Why is the ozone layer in danger? and What is the ozone hole? To learn more about the ozone layer, go to http://mocomi.com/what-is-the-ozo... -

AIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENT
-

Ozone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi Agrahari
Hope you enjoyed this video on Ozone Depletion by SuperProf Ravi Agrahari To watch more videos go to - http://bit.ly/1RTmgJf About the subject: Environment covers every living and non-living thing occurring naturally on Earth. Ecology is the scientific study of interactions between organisms and their environment. There are several practical applications of ecology in natural resource management, community health, economics, conservation biology and wetland management, among others. Biodiversity deals with different types of life found on earth. On Earth, biodiversity is not distributed evenly, rather it is the richest in the tropics. Studying Environment, Ecology and Bio-diversity is important for UPSC / IAS / IPS / IFS exam. About the Professor: Dr. Ravi P. Agrahari, Ph.D, IIT Delhi... -

Ozone Layer Danger
Think of the ozone layer as Earth's sunglasses, protecting life on the surface from the harmful glare of the sun's strongest ultraviolet rays, which can cause skin cancer and other maladies. Ozone stinks. People who breathe it gag as their lungs burn. The EPA classifies ground-level ozone as air pollution. Yet without it, life on Earth would be impossible. A fragile layer of ozone 25 km above Earth's surface is all that stands between us and some of the harshest UV rays from the sun. The ozone molecule O3 blocks radiation which would otherwise burn skin and cause cancer. On Mars, which has no ozone layer to protect it, solar UV rays strafe the surface with deadly effect, leaving the apparently lifeless planet without the simplest of organic molecules in the upper millimeters of exposed... -

-

What happened to the holes in the ozone layer? | Global Ideas
No sunbathing without sunscreen - that was a mantra for sun worshippers back in the 1980s. The reason: The sun's dangerous UV-radiation passes through massive holes in the Earth's ozone layer and strikes the Earth surface - and our skins - nearly unhindered. Today, we are still busy drenching ourselves in suncream, but it's got quiet about ozone holes. Just what happened to them? More information on: http://www.ideasforacoolerworld.org -

Causes of Ozone Depletion
Useful for CBSE, ICSE, NCERT & International Students Grade : 10 Subject : Biology Lesson :Our environment Topic: Causes of Ozone Depletion Ozone layer depletion, is simply the wearing out (reduction) of the amount of ozone in the stratosphere. Unlike pollution, which has many types and causes, Ozone depletion has been pinned down to one major human activity. Visit www.oztern.com to find personalized test preparation solutions for Pre Medical - AIPMT, AIIMS, JIPMER, State, Pre Engineering - IIT JEE, JEE MAIN, BITSAT, State and Foundations - Class 6 to 10. -

Ozone Depletion - Role of Halocarbons
Here's how halocarbons chew through ozone. Cl atoms react with an ozone molecule, but then the bare Cl atom is regenerated! -

NASA | Why is the Ozone Hole Getting Smaller?
For more information: http://www.nasa.gov/content/goddard/2014-antarctic-ozone-hole-holds-steady/#.VFvnQMdi-RI The Antarctic ozone hole reached its annual peak size on Sept. 11, according to scientists from NASA and the National Oceanic and Atmospheric Administration (NOAA). The size of this year’s hole was 24.1 million square kilometers (9.3 million square miles) — an area roughly the size of North America. With the increased atmospheric chlorine levels present since the 1980s, the Antarctic ozone hole forms and expands during the Southern Hemisphere spring (August and September). The ozone layer helps shield life on Earth from potentially harmful ultraviolet radiation that can cause skin cancer and damage plants. The Montreal Protocol agreement beginning in 1987 regulated ozone deple... -

Ozone Layer & Hole - Video for Kids
Visit http://www.makemegenius.com for more,free science videos for kids. Along-with beneficial rays from sun, there many harmful rays also which come from the sun.These harmful rays are known as ultraviolet rays ( uv rays) .If human skin is exposed too long to UV rays, it can cause cancer. But good news is that most of the harmful UV radiation is absorbed by a layer of ozone gas 20 -- 50 km above the Earth. But few human made chemicals are releasing few gases which are depleting the ozone layer.This video explains the whole ozone layer composition & depletion in an interesting way. -

NASA | Widely Used Coolants Contribute to Ozone Depletion
According to a new NASA study, a class of widely used chemical coolants known as hydrofluorocarbons (HFC), found in refrigerators and in home and automobile air conditioners, contributes to ozone depletion by a small but measurable amount, countering a decades-old assumption. This video is public domain and can be downloaded at: http://svs.gsfc.nasa.gov/cgi-bin/details.cgi?aid=12030 You can read more at: http://www.nasa.gov/press-release/goddard/nasa-study-shows-that-common-coolants-contribute-to-ozone-depletion Like our videos? Subscribe to NASA's Goddard Shorts HD podcast: http://svs.gsfc.nasa.gov/vis/iTunes/f0004_index.html Or find NASA Goddard Space Flight Center on Facebook: http://www.facebook.com/NASA.GSFC Or find us on Twitter: http://twitter.com/NASAGoddard -

-

Reducing ozone depletion
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of a number of substances believed to be responsible for ozone depletion. The phase out is planned via a timetable. For example, the substance hydro chlorofluorocarbon has a first deadline of 2015 and a final phase-out by 2030. In its daily work, UNIDO focuses on cost-effective ways to reduce ozone-depleting substances such as freons, halons and chlorofluorocarbons, in the areas of refrigeration, plastic foams, halons, solvents, fumigants and aerosol. Once the government of a developing country identifies a company that requires assistance in eliminating an ozone depleting substance that originates during the production cycle, it approaches UNIDO with a request to find a so... -

CFC's and Ozone Depletion
-- Created using PowToon -- Free sign up at http://www.powtoon.com/ . Make your own animated videos and animated presentations for free. PowToon is a free tool that allows you to develop cool animated clips and animated presentations for your website, office meeting, sales pitch, nonprofit fundraiser, product launch, video resume, or anything else you could use an animated explainer video. PowToon's animation templates help you create animated presentations and animated explainer videos from scratch. Anyone can produce awesome animations quickly with PowToon, without the cost or hassle other professional animation services require. -

Ozone Layer Depletion
This video is about Ozone Layer Depletion Sources Used: http://www.technologystudent.com/despro_flsh/ozone1.html Ryan, V. "The Ozone Layer - CFC Gases and Background." The Ozone Layer - CFC Gases and Background. Technologystudent.com, 2008. Web. 20 Apr. 2014. http://environment.nationalgeographic.com/environment/global-warming/ozone-depletion-overview/ National Geographic. "Ozone Depletion Information, Ozone Depletion Facts, Ozone Layer, Ozone Hole - National Geographic." National Geographic. National Geographic, n.d. Web. 20 Apr. 2014. http://science.howstuffworks.com/environmental/green-science/ozone-layer3.htm Howstuffworks. "How the Ozone Layer Works." HowStuffWorks. HowStuffWorks, n.d. Web. 20 Apr. 2014. http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Case_Studies%3A_Kinet... -

NASA | Exploring Ozone
Want more? Subscribe to NASA on iTunes! http://phobos.apple.com/WebObjects/MZStore.woa/wa/viewPodcast?id=283424434 This short video combines dynamic ozone visualizations with an interview with leading atmospheric NASA scientist, Dr. Paul Newman. Dr. Newman explains why ozone is important, he cites the ingredients that cause an ozone hole to form, and he remarks on the future of the ozone, pointing to exciting new areas of ozone research, including the role climate change will play in future years. For more information: http://www.nasa.gov/vision/earth/environment/ozone_resource_page.html -

5.6- Stratospheric Ozone Depletion
Complete lesson on TED Ed: http://ed.ted.com/on/oFTbbU60 A comprehensive overview spanning the sources of ODS, the chemistry of the ozone hole and its management with HCFC's, widespread compliance and responsible disposal. A brief cost/benefit analysis is included and the principle of common but differentiated responsibility is highlighted. Made specifically for the IB Environmental Systems and Societies course but a useful resource for all students of environmental management.
Ozone Depletion
- Order: Reorder
- Duration: 2:45
- Updated: 16 Feb 2013
- views: 25726
- published: 16 Feb 2013
- views: 25726
OZONE DEPLETION
- Order: Reorder
- Duration: 4:06
- Updated: 27 Sep 2008
- views: 97142
- published: 27 Sep 2008
- views: 97142
ozone depletion
- Order: Reorder
- Duration: 3:50
- Updated: 31 Aug 2015
- views: 613
- published: 31 Aug 2015
- views: 613
Ozone Depletion
- Order: Reorder
- Duration: 1:32
- Updated: 02 Jun 2009
- views: 28348
- published: 02 Jun 2009
- views: 28348
Ozone Layer and it's Depletion [Animation]
- Order: Reorder
- Duration: 1:23
- Updated: 16 Apr 2014
- views: 14464
- published: 16 Apr 2014
- views: 14464
The Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific Journey
- Order: Reorder
- Duration: 16:37
- Updated: 15 Sep 2011
- views: 82652
- published: 15 Sep 2011
- views: 82652
What is Ozone Layer? | Mocomi Kids
- Order: Reorder
- Duration: 3:00
- Updated: 06 Nov 2012
- views: 101183
- published: 06 Nov 2012
- views: 101183
AIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENT
- Order: Reorder
- Duration: 4:25
- Updated: 04 Dec 2014
- views: 2242
- published: 04 Dec 2014
- views: 2242
Ozone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi Agrahari
- Order: Reorder
- Duration: 30:30
- Updated: 15 Jun 2015
- views: 4593
- published: 15 Jun 2015
- views: 4593
Ozone Layer Danger
- Order: Reorder
- Duration: 4:18
- Updated: 01 Jun 2013
- views: 115893
- published: 01 Jun 2013
- views: 115893
How CFC's Deplete the Ozone Layer
- Order: Reorder
- Duration: 5:44
- Updated: 16 Jun 2014
- views: 18622
What happened to the holes in the ozone layer? | Global Ideas
- Order: Reorder
- Duration: 2:02
- Updated: 08 Feb 2013
- views: 21689
- published: 08 Feb 2013
- views: 21689
Causes of Ozone Depletion
- Order: Reorder
- Duration: 2:56
- Updated: 05 Dec 2015
- views: 100
- published: 05 Dec 2015
- views: 100
Ozone Depletion - Role of Halocarbons
- Order: Reorder
- Duration: 5:06
- Updated: 05 Jun 2012
- views: 1335
- published: 05 Jun 2012
- views: 1335
NASA | Why is the Ozone Hole Getting Smaller?
- Order: Reorder
- Duration: 1:23
- Updated: 07 Nov 2014
- views: 38326
- published: 07 Nov 2014
- views: 38326
Ozone Layer & Hole - Video for Kids
- Order: Reorder
- Duration: 1:57
- Updated: 25 Jul 2012
- views: 79002
- published: 25 Jul 2012
- views: 79002
NASA | Widely Used Coolants Contribute to Ozone Depletion
- Order: Reorder
- Duration: 1:57
- Updated: 22 Oct 2015
- views: 16898
- published: 22 Oct 2015
- views: 16898
Ozone Depletion vs Global Warming
- Order: Reorder
- Duration: 4:43
- Updated: 02 Jun 2012
- views: 962
Reducing ozone depletion
- Order: Reorder
- Duration: 5:02
- Updated: 25 Apr 2012
- views: 2345
- published: 25 Apr 2012
- views: 2345
CFC's and Ozone Depletion
- Order: Reorder
- Duration: 5:06
- Updated: 13 Oct 2014
- views: 326
- published: 13 Oct 2014
- views: 326
Ozone Layer Depletion
- Order: Reorder
- Duration: 2:01
- Updated: 30 Apr 2014
- views: 1310
- published: 30 Apr 2014
- views: 1310
NASA | Exploring Ozone
- Order: Reorder
- Duration: 2:21
- Updated: 19 Oct 2007
- views: 343957
- published: 19 Oct 2007
- views: 343957
5.6- Stratospheric Ozone Depletion
- Order: Reorder
- Duration: 18:51
- Updated: 31 Aug 2013
- views: 1975
- published: 31 Aug 2013
- views: 1975
- Playlist
- Chat
- Playlist
- Chat

Ozone Depletion
- Report rights infringement
- published: 16 Feb 2013
- views: 25726

OZONE DEPLETION
- Report rights infringement
- published: 27 Sep 2008
- views: 97142

ozone depletion
- Report rights infringement
- published: 31 Aug 2015
- views: 613

Ozone Depletion
- Report rights infringement
- published: 02 Jun 2009
- views: 28348

Ozone Layer and it's Depletion [Animation]
- Report rights infringement
- published: 16 Apr 2014
- views: 14464

The Antarctic Ozone Hole -- From Discovery to Recovery, a Scientific Journey
- Report rights infringement
- published: 15 Sep 2011
- views: 82652

What is Ozone Layer? | Mocomi Kids
- Report rights infringement
- published: 06 Nov 2012
- views: 101183

AIR POLLUTION - OZONE DEPLETION BY CFC GASES AND IT'S EFFECTS ON THE ENVIRONMENT
- Report rights infringement
- published: 04 Dec 2014
- views: 2242

Ozone Depletion (Environment, Ecology and Bio-diversity) for UPSC by SuperProf Ravi Agrahari
- Report rights infringement
- published: 15 Jun 2015
- views: 4593

Ozone Layer Danger
- Report rights infringement
- published: 01 Jun 2013
- views: 115893

How CFC's Deplete the Ozone Layer
- Report rights infringement
- published: 16 Jun 2014
- views: 18622

What happened to the holes in the ozone layer? | Global Ideas
- Report rights infringement
- published: 08 Feb 2013
- views: 21689

Causes of Ozone Depletion
- Report rights infringement
- published: 05 Dec 2015
- views: 100

Ozone Depletion - Role of Halocarbons
- Report rights infringement
- published: 05 Jun 2012
- views: 1335

British Airways flight hits UFO during landing at Heathrow
Edit Ars Technica 18 Apr 2016Earthquake kills 238 in Ecuador; emergency workers rush in
Edit The Oklahoman 17 Apr 2016Anti-Isis coalition 'preparing to launch final assault on Raqqa stronghold', US official says
Edit The Independent 17 Apr 2016Japan earthquake: 'Nearly 250,000 told to leave amid fear of tremors'
Edit BBC News 18 Apr 2016Manus Island detainees plead: anywhere but Papua New Guinea
Edit The Guardian 18 Apr 2016Building the Foundation for Environmental Transparency (American Architectural Manufacturers Association)
Edit Public Technologies 15 Apr 2016Countries Get Down to Business on Phasing Down HFCs
Edit Huffington Post 10 Apr 2016Anthony (Tony) Michael Whittaker - an appreciation (Green Party of England and Wales)
Edit Public Technologies 07 Apr 2016World Bank Debars Six Companies for One Year In Relation to Misconduct in an Environmental Project in East Asia (World Bank Group)
Edit Public Technologies 05 Apr 2016Media Invitation: Countries Discuss Reduction of Climate-Change-Inducing HFCs (UNEP - United Nations Environment Programme)
Edit Public Technologies 04 Apr 2016EU seeks progress on HFC amendment to Montreal Protocol at Geneva talks (European Commission - Directorate General for Climate Action)
Edit Public Technologies 04 Apr 2016The WSJ's Long Record of Protecting Polluters
Edit Huffington Post 03 Apr 2016» Blocket’s black list; five things you should never throw away (Schibsted ASA)
Edit Public Technologies 31 Mar 2016Tolba, former UN environment chief, dies at age 93
Edit Times Union 29 Mar 2016ARCA Recycling and ARCA Advanced Processing Register $1.7 Million in Carbon Offset Credits from Two ...
Edit Stockhouse 29 Mar 2016UH-Clear Lake online environmental science program nationally ranked (University of Houston - Clear Lake)
Edit Public Technologies 29 Mar 2016The Nitrogen Ticking Time Bomb
Edit Mercola 29 Mar 2016Sierra Leone News: Salone rated 3rd most vulnerable country to climate change- Haddiatou Jallow
Edit Awoko 24 Mar 2016- 1
- 2
- 3
- 4
- 5
- Next page »






