- published: 10 Jul 2010
- views: 1501
-
remove the playlistAlpha Process
- remove the playlistAlpha Process
- published: 29 Sep 2015
- views: 249
- published: 19 May 2015
- views: 334
- published: 04 Apr 2015
- views: 701
- published: 28 Jan 2014
- views: 2818
- published: 29 Jan 2016
- views: 16
- published: 24 Jun 2011
- views: 305
- published: 13 Jun 2014
- views: 244859
The alpha process (or alpha reactions) is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process. While the triple-alpha process only requires helium, once some carbon is present, other reactions that consume helium are possible:
, Q = 7.16 МeV
, Q = 4.73 МeV
, Q = 9.31 МeV
, Q = 9.98 МeV
, Q = 6.95 МeV
All these reactions have a very low rate and therefore do not contribute significantly to the energy production in stars; with elements heavier than neon (atomic number > 10) they occur even less easily due to the increasing Coulomb barrier.
Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of the mass of the helium nucleus (the alpha particle). Alpha elements are Z ≤ 22: (C, N), O, Ne, Mg, Si, S, Ar, Ca, Ti. They are synthesized by alpha-capture in the silicon fusing precursor state to Type II supernovae. Silicon and calcium are purely alpha process elements. Magnesium can be burned by proton capture reactions. As for oxygen, some authors[which?] consider it an alpha element, while others do not. Oxygen is surely an alpha element in low metallicity population II stars. It is produced in Type II supernovae and its enhancement is well correlated with an enhancement of other alpha process elements. Sometimes C and N are considered alpha process elements, since they are synthesized in nuclear alpha-capture reactions.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 1:15
1:15Teach Astronomy - Triple-Alpha Process
Teach Astronomy - Triple-Alpha ProcessTeach Astronomy - Triple-Alpha Process
http://www.teachastronomy.com/ The core of an evolving star like a red giant contracts until the temperature reaches roughly two hundred million Kelvin. At this point a new energy source is available from the fusion of helium nuclei by the triple alpha process. This is a two stage reaction. In the first stage two helium four nuclei combine to form a beryllium 8 nucleus with a photon, and in the second stage a beryllium 8 nucleus combines with a helium 4 nucleus to form a carbon 12 nucleus with a photon released. Beryllium is unstable, and so the decay of beryllium before it can combine with another helium nucleus reduces the efficiency but does not quench the process. In low mass stars the energy released can rapidly heat the core and cause what's called a helium flash. This can consume the helium fuel in only a few seconds although the effects are seen at the outer cool envelope of the star hundreds or thousands of years later and can last thousands of years. The Sun faces a helium flash roughly three hundred million years after it leaves the main sequence. -
 2:46
2:46Triple-alpha process - Video Learning - WizScience.com
Triple-alpha process - Video Learning - WizScience.comTriple-alpha process - Video Learning - WizScience.com
The "triple-alpha process" is a set of nuclear fusion reactions by which three helium-4 nuclei are transformed into carbon. Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores. The products of further nuclear fusion reactions of helium with hydrogen or another helium nucleus produce lithium-5 and beryllium-8 respectively, both of which are highly unstable and decay almost instantly back into smaller nuclei. When the star starts to run out of hydrogen to fuse, the core of the star begins to collapse until the central temperature rises to 10 8 K . At this point helium nuclei are fusing together faster than their product, beryllium-8, decays back into two helium nuclei. Once beryllium-8 is produced a little faster than it decays, the number of beryllium-8 nuclei in the stellar core increases to a large number. Then in its core there will be many beryllium-8 nuclei that can fuse with another helium nucleus to form carbon-12, which is stable: The net energy release of the process is . Because the triple-alpha process is unlikely, it needs a long time to produce much carbon. One consequence of this is that no significant amount of carbon was produced in the Big Bang because within minutes after the Big Bang, the temperature fell below the critical point for nuclear fusion. Ordinarily, the probability of the triple alpha process is extremely small. However, the beryllium-8 ground state has almost exactly the energy of two alpha particles. In the second step, 8 Be + 4 He has almost exactly the energy of an excited state of 12 C. These resonances greatly increase the probability that an incoming alpha particle will combine with beryllium-8 to form carbon. The existence of this resonance was predicted by Fred Hoyle before its actual observation, based on the physical necessity for it to exist, in order for carbon to be formed in stars. In turn, prediction and then discovery of this energy resonance and process gave very significant support to Hoyle's hypothesis of stellar nucleosynthesis, which posited that all chemical elements had originally been formed from hydrogen, the true primordial substance. Wiz Science™ is "the" learning channel for children and all ages. SUBSCRIBE TODAY Disclaimer: This video is for your information only. The author or publisher does not guarantee the accuracy of the content presented in this video. USE AT YOUR OWN RISK. Background Music: "The Place Inside" by Silent Partner (royalty-free) from YouTube Audio Library. This video uses material/images from https://en.wikipedia.org/wiki/Triple-alpha+process, which is released under Creative Commons Attribution-Share-Alike License 3.0 http://creativecommons.org/licenses/by-sa/3.0/ . This video is licensed under Creative Commons Attribution-Share-Alike License 3.0 http://creativecommons.org/licenses/by-sa/3.0/ . To reuse/adapt the content in your own work, you must comply with the license terms. -
 4:04
4:04Everyn - Alpha Process
Everyn - Alpha ProcessEveryn - Alpha Process
The fifth track from our self-titled EP (free download @ http://everyn.bandcamp.com/) Thanks for checking out our music! Make sure to listen in HD! Download: http://everyn.bandcamp.com/ http://facebook.com/Everynband Tracking & Mixing by Mike Krzeminski Mastering & Production by Josh Bodnar -
 4:55
4:55Alpha Process - Je T'aime
Alpha Process - Je T'aime -
 6:25
6:25Alpha Process - Je T'aime
Alpha Process - Je T'aime -
 6:01
6:01Alpha Process je t'aime
Alpha Process je t'aimeAlpha Process je t'aime
-
 36:27
36:27Solar H-alpha Processing With Photoshop
Solar H-alpha Processing With PhotoshopSolar H-alpha Processing With Photoshop
In this video I show most of the basic steps I take to process my Solar H-alpha images. I have put the RAW images in a folder for you to download to follow along and experiment with. https://www.dropbox.com/sh/xp5s20gsgza81i9/AABBeg7WZV5GIWk465MFVkC_a?dl=0 Here are some useful links on the web: https://www.youtube.com/user/Helium3Fusion http://www.photosbykev.com/wordpress/photography/pst-solar-imagin/ http://www.astro-nut.com/SolarPrimer.pdf The colour balance I used for my solar colour action is as follows: Shadows RED +50, GREEN +10, BLUE -100 Midtones RED +20, GREEN 0, BLUE -60 Highlights RED +30, GREEN -20, BLUE -40 -
 2:18
2:18Alpha Process Controls Marine ERC Coupling STS
Alpha Process Controls Marine ERC Coupling STSAlpha Process Controls Marine ERC Coupling STS
Alpha Process Control Marine Breakaway Couplings provide safety critical protection from spillage when ships or barges are transferring hazardous cargoes to shore. ERC breakaway coupling provides safety for plant and personnel in the event that a road, rail or ship moves off while still connected to the loading arm. This unique breakaway coupling is activated by a simple stainless steel pull cable that comes into tension a predetermined point before the loading arm exceeds it's normal working envelope. -
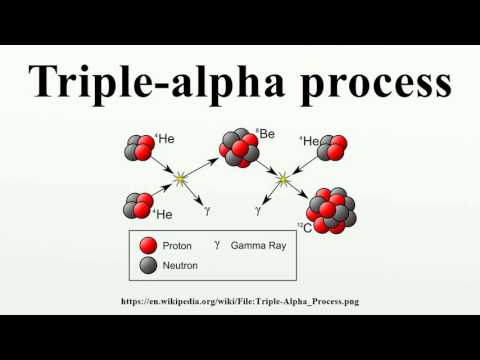 11:42
11:42Triple-alpha process
Triple-alpha processTriple-alpha process
Triple-alpha process The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores. =======Image-Copyright-Info======== License: Creative Commons Attribution-Share Alike 3.0 (CC-BY-SA-3.0) LicenseLink: http://creativecommons.org/licenses/by-sa/3.0/ Image Source: https://en.wikipedia.org/wiki/File:Triple-Alpha_Process.png =======Image-Copyright-Info======== -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=AS6R6CZQ7uc -
 1:18
1:18Triple Alpha Process
Triple Alpha Process -
 3:00
3:00Bestovitch - Triple Alpha Process EP
Bestovitch - Triple Alpha Process EPBestovitch - Triple Alpha Process EP
A VKRS Netlabel release -- Bestovitch -- Triple Alpha Process EP Bestovitch (aka Paul Best) was born in Dublin but has lived most of his life in Belfast. His first foray into electronic music scene was at the age of 16 as, he says, "the techno club in the city centre was the only one that would let us in at that age". It was there that classic acts such as Carl Cox, Dave Clarke and Leftfield acted as inspiration. Additional influences such as Aphex Twin and Squarepusher were the catalyst for his distinctive hardware-driven sound and Paul continues to collect and buy whichever synths and drum machines he can lay his hands on to create new sounds and patches. Consequently studio 'Bestovitch' is under a persistent threat of being overrun by cables, keyboards and boxes of sound but it's our humble opinion that he shouldn't have it any other way.To quote Paul directly "If it makes a bleep, bloop or percussive sound I'll use it". Download the EP from http://vkrs.co/netlabel/netlabel-releases-may-2011 -
 2:14
2:14Due Process: Alpha Trailer
Due Process: Alpha TrailerDue Process: Alpha Trailer
Check out http://dueprocess.info/ for our devblog! https://twitter.com/EnemyCrabGames https://www.facebook.com/dueprocessgame Music by ranikade3: https://soundcloud.com/ranikade3/snatch-theme-diamond https://www.youtube.com/user/ranikade3 Remember folks, as this is super alpha, visuals are very, placeholder. Due Process is a very indie (read: WIP) love letter to the "classic" tactical shooters of yore, like SWAT and the original Rainbow Six series. It's a strictly multiplayer game that focuses on cooperation, tactics, and planning. Players have 2 minutes at the start of a round to draw up a plan John Madden-style, involving tools like flashbangs, night vision goggles, and wall breaching charges to coordinate the perfect assault, while defenders scheme to predict and spoil it. These strategies collide in a bloody gunfight that ends in the blink of an eye. We're still very early (could you tell?), but you can put your name on the list for alpha testing here: signup@dueprocess.info -
 0:10
0:10Alpha Process Controls Cryogenic Breakaway Coupling Demonstration
Alpha Process Controls Cryogenic Breakaway Coupling Demonstration -
 20:45
20:45Due Process Early Alpha - Episode 1
Due Process Early Alpha - Episode 1Due Process Early Alpha - Episode 1
Huge shout out to Enemy Crab Games for getting in contact with us and allowing us to play and record this early edition of Due Process. All Info About The Game: Website: DueProcess.info Press Kit: DueProcess.info/press/sheet.php?p=due_process Facebook: facebook.com/DueProcessGame Twitter: twitter.com/EnemyCrabGames ---------------------------------------------------------------------------------- My Channel: http://www.youtube.com/user/PauseUnpause Suggest A Game: http://pauseunpause.tumblr.com/ask Watch Me Live: http://twitch.tv/PauseUnpause My Facebook: http://facebook.com/PauseUnpause My Twitter: https://twitter.com/#!/PauseUnpauses My Tumblr: http://pauseunpause.tumblr.com/ Mindcrack Podcast: http://youtube.com/MindcrackNetwork Donate: http://bit.ly/nU8Nre ---------------------------------------------------------------------------------- And of course, if you enjoyed the video, don't forget to like, favorite and subscribe!
- Accretion disc
- Alpha decay
- Alpha particle
- Alpha process
- Am star
- Ap and Bp stars
- Apparent magnitude
- Argon
- Asterism (astronomy)
- Asteroseismology
- Atomic number
- Barium star
- Be star
- Beta decay
- Binary star
- Black dwarf
- Blue giant
- Blue straggler
- Blue supergiant
- Bok globule
- Boson star
- Bright giant
- Brown dwarf
- Calcium
- Carbon
- Carbon star
- CH star
- Chromium
- Chromosphere
- Circumpolar star
- Cluster decay
- CNO cycle
- Cobalt
- Color–color diagram
- Common envelope
- Compact star
- Constellation
- Contact binary
- Convection zone
- Corona
- Coulomb barrier
- Double beta decay
- Dredge-up
- Dwarf star
- Eddington luminosity
- EF Eridani
- Electron capture
- Exotic star
- Extreme helium star
- FU Orionis star
- Fusor (astronomy)
- Galaxy
- Gamma ray
- Giant star
- Globular cluster
- Gravitation
- H II region
- Hayashi limit
- Hayashi track
- Helioseismology
- Helium
- Helium flash
- Helium planet
- Henyey track
- Herbig Ae Be star
- Herbig–Haro object
- Horizontal branch
- Hypergiant
- Hypernova
- Infrared dark cloud
- Instability strip
- Intergalactic star
- Internal conversion
- Iron
- Iron peak
- Iron star
- Isomeric transition
- Lambda Boötis star
- Late-type star
- Lead star
- List of brown dwarfs
- List of novae
- List of supernovae
- Lists of stars
- Lithium burning
- Luminous red nova
- Magnesium
- Magnetar
- Main sequence
- Manganese
- Metallicity
- Microturbulence
- Mira variable
- Molecular cloud
- Multiple star
- Neon
- Neon-burning process
- Neutron capture
- Neutron emission
- Neutron star
- Nickel
- Nitrogen
- Nova
- Nova remnant
- Nuclear fusion
- OB star
- Open cluster
- Orion variable
- Oxygen
- P-process
- Parallax
- Peculiar star
- PG 1159 star
- Photosphere
- Planet
- Planetar (astronomy)
- Planetary nebula
- Planetary system
- Pole star
- Population II star
- Portal Star
- Positron emission
- Proper motion
- Proton emission
- Protostar
- Pulsar
- Quark star
- Quasi-star
- R-process
- Radial velocity
- Radiation zone
- Radioactive decay
- Red clump
- Red dwarf
- Red giant
- Red supergiant
- Rp-process
- S-process
- S-type star
- Shell star
- Silicon
- Solar core
- Solar System
- Spontaneous fission
- Star
- Star cluster
- Star designation
- Star formation
- Star system
- Starspot
- Stellar association
- Stellar atmosphere
- Stellar black hole
- Stellar dynamics
- Stellar evolution
- Stellar kinematics
- Stellar mass
- Stellar rotation
- Stellar structure
- Stellar wind
- Stellar-wind bubble
- Sub-brown dwarf
- Subdwarf B star
- Subdwarf star
- Subgiant
- Substellar object
- Sulfur
- Supercluster
- Supergiant
- Supernova
- Supernova impostor
- T Tauri star
- Technetium star
- Template Star
- Template talk Star
- Thorne–Żytkow object
- Titanium
- Triple-alpha process
- Type Ia supernova
- Type II supernova
- Vanadium
- Variable star
- White dwarf
- Wolf–Rayet star
- Yellow hypergiant
- Yellow supergiant
- Young stellar object
-

Teach Astronomy - Triple-Alpha Process
http://www.teachastronomy.com/ The core of an evolving star like a red giant contracts until the temperature reaches roughly two hundred million Kelvin. At this point a new energy source is available from the fusion of helium nuclei by the triple alpha process. This is a two stage reaction. In the first stage two helium four nuclei combine to form a beryllium 8 nucleus with a photon, and in the second stage a beryllium 8 nucleus combines with a helium 4 nucleus to form a carbon 12 nucleus with a photon released. Beryllium is unstable, and so the decay of beryllium before it can combine with another helium nucleus reduces the efficiency but does not quench the process. In low mass stars the energy released can rapidly heat the core and cause what's called a helium flash. This can cons... -

Triple-alpha process - Video Learning - WizScience.com
The "triple-alpha process" is a set of nuclear fusion reactions by which three helium-4 nuclei are transformed into carbon. Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores. The products of further nuclear fusion reactions of helium with hydrogen or another helium nucleus produce lithium-5 and beryllium-8 respectively, both of which are highly unstable and decay almost instantly back into smaller nuclei. When the star starts to run out of hydrogen to fuse, the core of the star begins to collapse until the central temperature rises to 10 8 K . At this point helium nuclei are fusing together faster than their product, beryllium-8, decays back into two helium nuclei. Once beryllium-8 is produced a... -

Everyn - Alpha Process
The fifth track from our self-titled EP (free download @ http://everyn.bandcamp.com/) Thanks for checking out our music! Make sure to listen in HD! Download: http://everyn.bandcamp.com/ http://facebook.com/Everynband Tracking & Mixing by Mike Krzeminski Mastering & Production by Josh Bodnar -

-

-

Alpha Process je t'aime
-

Solar H-alpha Processing With Photoshop
In this video I show most of the basic steps I take to process my Solar H-alpha images. I have put the RAW images in a folder for you to download to follow along and experiment with. https://www.dropbox.com/sh/xp5s20gsgza81i9/AABBeg7WZV5GIWk465MFVkC_a?dl=0 Here are some useful links on the web: https://www.youtube.com/user/Helium3Fusion http://www.photosbykev.com/wordpress/photography/pst-solar-imagin/ http://www.astro-nut.com/SolarPrimer.pdf The colour balance I used for my solar colour action is as follows: Shadows RED +50, GREEN +10, BLUE -100 Midtones RED +20, GREEN 0, BLUE -60 Highlights RED +30, GREEN -20, BLUE -40 -

Alpha Process Controls Marine ERC Coupling STS
Alpha Process Control Marine Breakaway Couplings provide safety critical protection from spillage when ships or barges are transferring hazardous cargoes to shore. ERC breakaway coupling provides safety for plant and personnel in the event that a road, rail or ship moves off while still connected to the loading arm. This unique breakaway coupling is activated by a simple stainless steel pull cable that comes into tension a predetermined point before the loading arm exceeds it's normal working envelope. -

Triple-alpha process
Triple-alpha process The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores. =======Image-Copyright-Info======== License: Creative Commons Attribution-Share Alike 3.0 (CC-BY-SA-3.0) LicenseLink: http://creativecommons.org/licenses/by-sa/3.0/ Image Source: https://en.wikipedia.org/wiki/File:Triple-Alpha_Process.png =======Image-Copyright-Info======== -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=AS6R6CZQ7uc -

-

Bestovitch - Triple Alpha Process EP
A VKRS Netlabel release -- Bestovitch -- Triple Alpha Process EP Bestovitch (aka Paul Best) was born in Dublin but has lived most of his life in Belfast. His first foray into electronic music scene was at the age of 16 as, he says, "the techno club in the city centre was the only one that would let us in at that age". It was there that classic acts such as Carl Cox, Dave Clarke and Leftfield acted as inspiration. Additional influences such as Aphex Twin and Squarepusher were the catalyst for his distinctive hardware-driven sound and Paul continues to collect and buy whichever synths and drum machines he can lay his hands on to create new sounds and patches. Consequently studio 'Bestovitch' is under a persistent threat of being overrun by cables, keyboards and boxes of sound but it's our... -

Due Process: Alpha Trailer
Check out http://dueprocess.info/ for our devblog! https://twitter.com/EnemyCrabGames https://www.facebook.com/dueprocessgame Music by ranikade3: https://soundcloud.com/ranikade3/snatch-theme-diamond https://www.youtube.com/user/ranikade3 Remember folks, as this is super alpha, visuals are very, placeholder. Due Process is a very indie (read: WIP) love letter to the "classic" tactical shooters of yore, like SWAT and the original Rainbow Six series. It's a strictly multiplayer game that focuses on cooperation, tactics, and planning. Players have 2 minutes at the start of a round to draw up a plan John Madden-style, involving tools like flashbangs, night vision goggles, and wall breaching charges to coordinate the perfect assault, while defenders scheme to predict and spoil it. These stra... -

-

Due Process Early Alpha - Episode 1
Huge shout out to Enemy Crab Games for getting in contact with us and allowing us to play and record this early edition of Due Process. All Info About The Game: Website: DueProcess.info Press Kit: DueProcess.info/press/sheet.php?p=due_process Facebook: facebook.com/DueProcessGame Twitter: twitter.com/EnemyCrabGames ---------------------------------------------------------------------------------- My Channel: http://www.youtube.com/user/PauseUnpause Suggest A Game: http://pauseunpause.tumblr.com/ask Watch Me Live: http://twitch.tv/PauseUnpause My Facebook: http://facebook.com/PauseUnpause My Twitter: https://twitter.com/#!/PauseUnpauses My Tumblr: http://pauseunpause.tumblr.com/ Mindcrack Podcast: http://youtube.com/MindcrackNetwork Donate: http://bit.ly/nU8Nre -------------------... -

Due Process Early Alpha - Episode 2
Huge shout out to Enemy Crab Games for getting in contact with us and allowing us to play and record this early edition of Due Process. All Info About The Game: Website: DueProcess.info Press Kit: DueProcess.info/press/sheet.php?p=due_process Facebook: facebook.com/DueProcessGame Twitter: twitter.com/EnemyCrabGames ---------------------------------------------------------------------------------- My Channel: http://www.youtube.com/user/PauseUnpause Suggest A Game: http://pauseunpause.tumblr.com/ask Watch Me Live: http://twitch.tv/PauseUnpause My Facebook: http://facebook.com/PauseUnpause My Twitter: https://twitter.com/#!/PauseUnpauses My Tumblr: http://pauseunpause.tumblr.com/ Mindcrack Podcast: http://youtube.com/MindcrackNetwork Donate: http://bit.ly/nU8Nre -------------------... -

Due Process - Part 1: "Attack and Defend!" (Pre-Alpha Gameplay)
Due Process. Welcome to Due Process an attack and defend style SWAT shooter where you either defend a randomly generated building or attack said building. Use everything at your disposal and plan everything. This game requires great teamwork and gun skill in order to pull off a round. Leave a like if you enjoyed :D Information: Facebook: facebook.com/dueprocessgame Twitter: twitter.com/EnemyCrabGames Website: dueprocess.info/ People in this video: Star: https://www.youtube.com/user/TheApocalypticStudio Fuzzy: https://www.youtube.com/user/EireBornFenix Baron: https://www.youtube.com/user/BaronVonGamez Social Media: ☆ Twitter: https://twitter.com/PartiallyRoyal ☆ Facebook: https://www.facebook.com/PartiallyRoyal ☆ Livestream: http://www.twitch.tv/esgame ☆ Cheap Games: https://www.g2... -

-

Due Process | Alpha Gameplay #1 - Getting Started
"Due Process is a very alpha love letter to "classic" tactical shooters of yore, like SWAT and the Rainbow Six series. It's a strictly multiplayer game that focuses on cooperation, tactics, and planning. Players have 2 minutes at the start of a round to draw up a plan John Madden-style, making use of tools like breaching charges, riot shields, and night vision goggles to coordinate the perfect assault, while defenders try to anticipate and foil their plans. These strategies collide in a bloody gunfight that ends in the blink of an eye." Join myself, some friends, and the devs in playing an alpha build of DUE PROCESS, a highly addictive tactical shooter! ~*Featuring*~ Pause: http://youtube.com/PauseUnpause Coestar: http://youtube.com/Coestar Millbee: http://youtube.com/MillBeeful W92Baj: ... -

Due Process - SWAT Showdown - LAN Party
THIS GAME IS PRE-ALPHA: Which means the build we are playing is no where near complete. It is not available for download to the public. Due Process is a tactical first person shooter inspired by SWAT team tactics. We got a chance to play and early alpha build with the Dev's in Seattle and it's an absolute blast! Check out more about the game on their site below! http://dueprocess.info/ What game should we play next? Let us know! Don't forget to like/follow us on Facebook & Twitter! http://facebook.com/node http://twitter.com/nodestudios http://twitter.com/cerberusarms http://twitter.com/corridordigital http://facebook.com/corridordigital http://twitter.com/fwong http://twitter.com/brandonjla http://facebook.com/freddiewspage -

Alpha Racing BMW S 1000 RR Building Process
Alpha Racing BMW S 1000 RR Building Process (Assembly Line) Subscribe https://www.youtube.com/user/myDriftFun?sub_confirmation=1 Subscribe to our google plus https://plus.google.com/u/0/b/113277730106072861939/+myDriftFun/posts Latest Car News - http://mydriftfun.com -

Alpha Process - Je T'aime.wmv
-

Due Process Gameplay Part 1 - Squad-Based Tactical Shooter! (Pre-Alpha)
Due Process is a tactical shooter game where the gameplay revolves around the Police/SWAT having to infiltrate a base that the criminals are holding down. Before every round, each team gets a planning phase where they can draw out where people should hold down, what they should watch, where they should come in from, what should be exploded, etc. Every round, everybody gets one life each, and once you die, you're dead for that round. There are 2 ways for the criminals to win, by killing everyone on the opposing team, or by surviving until the time limit runs out. The Police can only win through killing the opposing team (at this point in development). The Police force also has much better weaponry along with being armed with flashbangs, explosives, etc. In Part 1 of our Due Process Gameplay... -

Alpha process
Alpha process The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.While the triple-alpha process only requires helium, once some carbon is present, these other reactions that consume helium are possible: , E = 7.16 МeV , E = 4.73 МeV , E = 9.32 МeV , E = 9.98 МeV , E = 6.95 МeV , E = 6.64 МeV , E = 7.04 МeV , E = 5.13 МeV , E = 7.70 МeV , E = 7.94 МeV , E = 8.00 МeV E is the energy produced by the reaction, released primarily as gamma rays (). -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=SlfJ29L5Nl4
Teach Astronomy - Triple-Alpha Process
- Order: Reorder
- Duration: 1:15
- Updated: 10 Jul 2010
- views: 1501
- published: 10 Jul 2010
- views: 1501
Triple-alpha process - Video Learning - WizScience.com
- Order: Reorder
- Duration: 2:46
- Updated: 29 Sep 2015
- views: 249
- published: 29 Sep 2015
- views: 249
Everyn - Alpha Process
- Order: Reorder
- Duration: 4:04
- Updated: 19 May 2015
- views: 334
- published: 19 May 2015
- views: 334
Alpha Process - Je T'aime
- Order: Reorder
- Duration: 4:55
- Updated: 03 Apr 2009
- views: 23034
Alpha Process - Je T'aime
- Order: Reorder
- Duration: 6:25
- Updated: 10 Jan 2015
- views: 554
Alpha Process je t'aime
- Order: Reorder
- Duration: 6:01
- Updated: 29 Jul 2010
- views: 1159
- published: 29 Jul 2010
- views: 1159
Solar H-alpha Processing With Photoshop
- Order: Reorder
- Duration: 36:27
- Updated: 04 Apr 2015
- views: 701
- published: 04 Apr 2015
- views: 701
Alpha Process Controls Marine ERC Coupling STS
- Order: Reorder
- Duration: 2:18
- Updated: 28 Jan 2014
- views: 2818
- published: 28 Jan 2014
- views: 2818
Triple-alpha process
- Order: Reorder
- Duration: 11:42
- Updated: 29 Jan 2016
- views: 16
- published: 29 Jan 2016
- views: 16
Triple Alpha Process
- Order: Reorder
- Duration: 1:18
- Updated: 10 Jan 2015
- views: 41
Bestovitch - Triple Alpha Process EP
- Order: Reorder
- Duration: 3:00
- Updated: 24 Jun 2011
- views: 305
- published: 24 Jun 2011
- views: 305
Due Process: Alpha Trailer
- Order: Reorder
- Duration: 2:14
- Updated: 13 Jun 2014
- views: 244859
- published: 13 Jun 2014
- views: 244859
Alpha Process Controls Cryogenic Breakaway Coupling Demonstration
- Order: Reorder
- Duration: 0:10
- Updated: 20 Feb 2014
- views: 161
Due Process Early Alpha - Episode 1
- Order: Reorder
- Duration: 20:45
- Updated: 16 Jan 2015
- views: 40100
- published: 16 Jan 2015
- views: 40100
Due Process Early Alpha - Episode 2
- Order: Reorder
- Duration: 16:57
- Updated: 20 Jan 2015
- views: 29245
- published: 20 Jan 2015
- views: 29245
Due Process - Part 1: "Attack and Defend!" (Pre-Alpha Gameplay)
- Order: Reorder
- Duration: 18:54
- Updated: 28 Sep 2014
- views: 110639
- published: 28 Sep 2014
- views: 110639
Alpha Process - Je t'aime (Kris Curvers 2015 Rework)
- Order: Reorder
- Duration: 5:52
- Updated: 26 Jul 2015
- views: 71
Due Process | Alpha Gameplay #1 - Getting Started
- Order: Reorder
- Duration: 19:55
- Updated: 16 Jan 2015
- views: 5090
- published: 16 Jan 2015
- views: 5090
Due Process - SWAT Showdown - LAN Party
- Order: Reorder
- Duration: 11:36
- Updated: 15 Aug 2014
- views: 728027
- published: 15 Aug 2014
- views: 728027
Alpha Racing BMW S 1000 RR Building Process
- Order: Reorder
- Duration: 16:55
- Updated: 03 Mar 2015
- views: 19667
- published: 03 Mar 2015
- views: 19667
Alpha Process - Je T'aime.wmv
- Order: Reorder
- Duration: 6:00
- Updated: 23 Dec 2010
- views: 4701
- published: 23 Dec 2010
- views: 4701
Due Process Gameplay Part 1 - Squad-Based Tactical Shooter! (Pre-Alpha)
- Order: Reorder
- Duration: 16:05
- Updated: 28 Sep 2014
- views: 8252
- published: 28 Sep 2014
- views: 8252
Alpha process
- Order: Reorder
- Duration: 2:50
- Updated: 22 Jan 2016
- views: 6
- published: 22 Jan 2016
- views: 6
- Playlist
- Chat
- Playlist
- Chat

Teach Astronomy - Triple-Alpha Process
- Report rights infringement
- published: 10 Jul 2010
- views: 1501

Triple-alpha process - Video Learning - WizScience.com
- Report rights infringement
- published: 29 Sep 2015
- views: 249

Everyn - Alpha Process
- Report rights infringement
- published: 19 May 2015
- views: 334

Alpha Process - Je T'aime
- Report rights infringement
- published: 03 Apr 2009
- views: 23034


Alpha Process je t'aime
- Report rights infringement
- published: 29 Jul 2010
- views: 1159

Solar H-alpha Processing With Photoshop
- Report rights infringement
- published: 04 Apr 2015
- views: 701

Alpha Process Controls Marine ERC Coupling STS
- Report rights infringement
- published: 28 Jan 2014
- views: 2818

Triple-alpha process
- Report rights infringement
- published: 29 Jan 2016
- views: 16

Triple Alpha Process
- Report rights infringement
- published: 10 Jan 2015
- views: 41

Bestovitch - Triple Alpha Process EP
- Report rights infringement
- published: 24 Jun 2011
- views: 305

Due Process: Alpha Trailer
- Report rights infringement
- published: 13 Jun 2014
- views: 244859

Alpha Process Controls Cryogenic Breakaway Coupling Demonstration
- Report rights infringement
- published: 20 Feb 2014
- views: 161

Due Process Early Alpha - Episode 1
- Report rights infringement
- published: 16 Jan 2015
- views: 40100
'Adorable' Prince George wows Obama in pyjamas
Edit Deccan Herald 23 Apr 20163 kids survive slaying of 8 family members in Ohio
Edit CNN 23 Apr 2016Saudi Arabia may be in for a nasty shock when Obama steps down
Edit The Independent 22 Apr 2016North Korea 'fires submarine-launched ballistic missile'
Edit BBC News 23 Apr 2016Donald Trump mimicks Indian call-center worker
Edit Asia Times 23 Apr 2016Global Methyl Methacrylate (MMA) Industry
Edit PR Newswire 01 Mar 2016Morgan Stanley Wealth Management Launches New Manager Analysis / Scoring Process for Fixed Income (Morgan ...
Edit Public Technologies 16 Feb 2016Financial Engineering Seminar: Lee Dicker "Multistage Portfolio Optimization with Transaction Costs and Parameter Uncertainty" (Stevens ...
Edit Public Technologies 22 Oct 2015An undervalued biotech is testing the cure for cancer now, and Chen Lin wants in
Edit Stockhouse 03 Jun 2015Lessons (Un)Learned in the Last 5 Years in Offshore Oil Industry Since the BP Deepwater ...
Edit Huffington Post 22 Apr 2015Green economy priorities
Edit Sun Star 16 Mar 2015Janney Montgomery Scott Wins StarMine Analyst Awards
Edit Business Wire 13 Oct 2014STEM Startups in Southeastern Connecticut Gather on Sept. 4 in Groton to Discuss Accelerating Community ...
Edit San Francisco Chronicle 04 Sep 2014Strange Supernova Casts Doubt On Star Explosion Theories
Edit Huffington Post 01 Aug 2014OU students publish national journal on politics (Oakland University)
Edit noodls 04 Apr 2014Researchers Describe Oxygen’s Different Shapes (North Carolina State University)
Edit noodls 13 Mar 2014- 1
- 2
- 3
- 4
- 5
- Next page »







