- published: 07 Oct 2013
- views: 18097
Create your page here
-
remove the playlist1765
- remove the playlist1765
back to playlist
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occurred in history!
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom.
According to the report, Bahraini security forces arrested one thousand seven hundred and sixty five people last year. The detainees included one hundred and twenty children. The report says there was a total ban on gathering and demonstrations across Bahrain in 2015. And the crackdown on protesters left over 700 people injured. The report was prepared by the Liberties and Human Rights Department of al-Wefaq National Islamic Society. Bahrain has been witnessing protests on a daily basis since the popular uprising began in the kingdom in February 2011. The crackdown on demonstrators has left many people dead.
Colin Cavell
Author and Lecturer
Watch Live: http://www.presstv.ir/live.html
Twitter: http://twitter.com/PressTV
LiveLeak: http://www.liveleak.com/c/PressTV
Facebook: http://www.facebook.com/PRESSTV
Google+: http://plus.google.com/+VideosPTV
Instagram: http://instagram.com/presstvchannel
SoundCloud: https://soundcloud.com/videosptv
- published: 11 May 2016
- views: 30
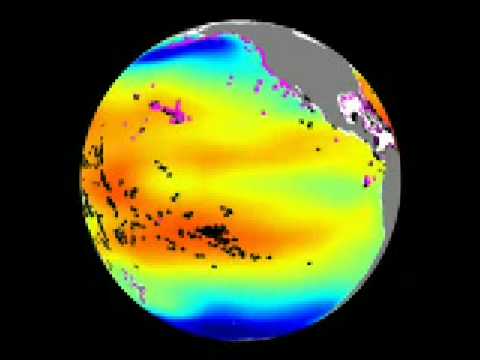
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in the ocean. For organisms with external calcium carbonate skeletons, such as corals and pteropods, the consequences can be very negative if the oceans become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. It has been demonstrated that pteropods, a planktonic mollusk, may not be able to maintain their shells in undersaturated waters. In some studies, live pteropods were subjected to waters that are undersaturated with respect to aragonite and their shells begin to dissolve within 48 hours (Feely et al., 2004; Orr et al 2005 ).
Image of Ocean Acidification Color Bar
This dataset has both historic and forecasted model-based changes in the ocean's carbonate chemistry due to increasing CO2 levels, as well as the presence of coral reefs depicted. The coral reefs are represented by black X's for shallow water species and magenta X's for deep water species. The ocean acidification levels are based on the median model output of the 13 international ocean carbon models based on the OCMIP-2 Project (Orr et al. 2005). The model outputs are represented on the global map in 5-yr increments from 1765 thru 2100. The future changes in CO2 chemistry were determined based on atmospheric CO2 scenarios presented in The Intergovernmental Panel on Climate Change (IPCC) IS92a 'continually increasing' CO2 emissions scenario (788 p.p.m.v. in the year 2100). As atmospheric levels of CO2 increase, the amount of CO2 that is absorbed by the ocean increases as well. This increase of CO2 in the ocean upsets the carbonate chemistry of the ocean and causes the waters to become more corrosive. The images indicate that all of the Southern Ocean surface waters south of 60°S and portions of the North Pacific become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. The scale to the left is proportional to the level of aragonite present in the ocean.
- published: 17 Dec 2008
- views: 9377
For more details:
http://view.paradym.com/3814731
1765 Glenridge Drive
Kernersville, NC 27284
$268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299
Immaculate and open w/all the bells & whistles, lightly lived in. Elegant kitchen cabinets, granite countertops, hardwood floors. Generous master and suite, comfortable living area w/beautiful fireplace & gas logs, 2 bedrooms with a full bath, & a wonderful sunroom on 1st floor. The upper level offers a 4th bedroom & full bath. Tinted windows, ceiling fans throughout. Beautifully manicured lawn, concrete landscape border& decorative wrought iron fence surrounds the backyard.
Presented By:
Vicki Crutchfield, Coldwell Banker Triad, Realtors
336-362-9412
View My Inventory:
http://my.paradym.com/inventory.asp?u=242251
- published: 11 May 2016
- views: 0
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica.
- published: 20 Aug 2008
- views: 256265
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 Other homes for sale by Joe Jenkins of (256010008) COLDWELL BANKER SUNSTAR REALTY: http://www.propertypanorama.com/all-tours/253242 Lowest Priced 3 Bedroom, 2 Bath Condo On Manasota Key, And It Is A Direct Bay Front Townhouse. Outstanding Full Bay Views From The Living & Dining Room Areas As Well As The Kitchen And Master Bedroom & There Are Even Partial Gulf Views From The 2Nd Bedroom. It Has A Good Rental History Of Over $21,000 Annually And That Is After The Owners Block It Out From Time To Time For Personal Use Or For Friends Or Other Family ...
- published: 09 May 2016
- views: 1
Year 1765 (MDCCLXV) was a common year starting on Tuesday (link will display the full calendar) of the Gregorian calendar and a common year starting on Saturday of the 11-day slower Julian calendar.
This page contains text from Wikipedia, the Free Encyclopedia -
http://en.wikipedia.org/wiki/1765
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 12:40
12:40Stamp Act of 1765
Stamp Act of 1765Stamp Act of 1765
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occurred in history! -
 10:58
10:58(Arabian riding 1765) شرح عام و تركيب الشكيمة
(Arabian riding 1765) شرح عام و تركيب الشكيمة -
 5:26
5:26Report: Bahrain forces detained 1765 people in 2015
Report: Bahrain forces detained 1765 people in 2015Report: Bahrain forces detained 1765 people in 2015
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom. According to the report, Bahraini security forces arrested one thousand seven hundred and sixty five people last year. The detainees included one hundred and twenty children. The report says there was a total ban on gathering and demonstrations across Bahrain in 2015. And the crackdown on protesters left over 700 people injured. The report was prepared by the Liberties and Human Rights Department of al-Wefaq National Islamic Society. Bahrain has been witnessing protests on a daily basis since the popular uprising began in the kingdom in February 2011. The crackdown on demonstrators has left many people dead. Colin Cavell Author and Lecturer Watch Live: http://www.presstv.ir/live.html Twitter: http://twitter.com/PressTV LiveLeak: http://www.liveleak.com/c/PressTV Facebook: http://www.facebook.com/PRESSTV Google+: http://plus.google.com/+VideosPTV Instagram: http://instagram.com/presstvchannel SoundCloud: https://soundcloud.com/videosptv -
 57:45
57:45LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL.
LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL. -
 0:34
0:34Ocean Acidification 1765 - 2100
Ocean Acidification 1765 - 2100Ocean Acidification 1765 - 2100
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in the ocean. For organisms with external calcium carbonate skeletons, such as corals and pteropods, the consequences can be very negative if the oceans become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. It has been demonstrated that pteropods, a planktonic mollusk, may not be able to maintain their shells in undersaturated waters. In some studies, live pteropods were subjected to waters that are undersaturated with respect to aragonite and their shells begin to dissolve within 48 hours (Feely et al., 2004; Orr et al 2005 ). Image of Ocean Acidification Color Bar This dataset has both historic and forecasted model-based changes in the ocean's carbonate chemistry due to increasing CO2 levels, as well as the presence of coral reefs depicted. The coral reefs are represented by black X's for shallow water species and magenta X's for deep water species. The ocean acidification levels are based on the median model output of the 13 international ocean carbon models based on the OCMIP-2 Project (Orr et al. 2005). The model outputs are represented on the global map in 5-yr increments from 1765 thru 2100. The future changes in CO2 chemistry were determined based on atmospheric CO2 scenarios presented in The Intergovernmental Panel on Climate Change (IPCC) IS92a 'continually increasing' CO2 emissions scenario (788 p.p.m.v. in the year 2100). As atmospheric levels of CO2 increase, the amount of CO2 that is absorbed by the ocean increases as well. This increase of CO2 in the ocean upsets the carbonate chemistry of the ocean and causes the waters to become more corrosive. The images indicate that all of the Southern Ocean surface waters south of 60°S and portions of the North Pacific become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. The scale to the left is proportional to the level of aragonite present in the ocean. -
 3:40
3:401765 Glenridge Drive
1765 Glenridge Drive1765 Glenridge Drive
For more details: http://view.paradym.com/3814731 1765 Glenridge Drive Kernersville, NC 27284 $268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299 Immaculate and open w/all the bells & whistles, lightly lived in. Elegant kitchen cabinets, granite countertops, hardwood floors. Generous master and suite, comfortable living area w/beautiful fireplace & gas logs, 2 bedrooms with a full bath, & a wonderful sunroom on 1st floor. The upper level offers a 4th bedroom & full bath. Tinted windows, ceiling fans throughout. Beautifully manicured lawn, concrete landscape border& decorative wrought iron fence surrounds the backyard. Presented By: Vicki Crutchfield, Coldwell Banker Triad, Realtors 336-362-9412 View My Inventory: http://my.paradym.com/inventory.asp?u=242251 -
 0:25
0:251765 Rosewood Ln Ishpeming, MI 49849
1765 Rosewood Ln Ishpeming, MI 49849 -
 0:18
0:181765 Rosewood Ln Ishpeming, MI 49849
1765 Rosewood Ln Ishpeming, MI 49849 -
 5:30
5:30RFX/ Algunos tienen poca memoria histórica (1765)
RFX/ Algunos tienen poca memoria histórica (1765)RFX/ Algunos tienen poca memoria histórica (1765)
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica. -
 1:35
1:35RELAX... HAMPI 7 BLN... 0813 1765 7041
RELAX... HAMPI 7 BLN... 0813 1765 7041RELAX... HAMPI 7 BLN... 0813 1765 7041
-
 1:54
1:54WIDO PKL... 3,1 KG.. 0813 1765 7041
WIDO PKL... 3,1 KG.. 0813 1765 7041WIDO PKL... 3,1 KG.. 0813 1765 7041
-
 1:59
1:591765 Gulf Blvd #202, Englewood, FL 34223
1765 Gulf Blvd #202, Englewood, FL 342231765 Gulf Blvd #202, Englewood, FL 34223
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 Other homes for sale by Joe Jenkins of (256010008) COLDWELL BANKER SUNSTAR REALTY: http://www.propertypanorama.com/all-tours/253242 Lowest Priced 3 Bedroom, 2 Bath Condo On Manasota Key, And It Is A Direct Bay Front Townhouse. Outstanding Full Bay Views From The Living & Dining Room Areas As Well As The Kitchen And Master Bedroom & There Are Even Partial Gulf Views From The 2Nd Bedroom. It Has A Good Rental History Of Over $21,000 Annually And That Is After The Owners Block It Out From Time To Time For Personal Use Or For Friends Or Other Family ...
- 1683
- 1687
- 1688
- 1689
- 1691
- 1698
- 1705
- 1708
- 1711
- 1713
- 1719
- 1721
- 1725
- 1730s
- 1740s
- 1750s
- 1760s
- 1762
- 1763
- 1764
- 1765
- 1765 in archaeology
- 1765 in architecture
- 1765 in art
- 1765 in Canada
- 1765 in literature
- 1765 in music
- 1765 in poetry
- 1765 in science
- 1766
- 1767
- 1768
- 1770s
- 1780s
- 1790s
- 17th century
- 1810
- 1812
- 1813
- 1815
- 1818
- 1819
- 1825
- 1831
- 1832
- 1833
- 1837
- 1840
- 1841
- 1844
- 1846
- 1847
- 18th century
- 19th century
- 2nd millennium
- Ab urbe condita
- Abigail Adams
- Abigail Adams Smith
- Abigail Williams
- Andrew Oliver
- Antoine de Beauterne
- April 1
- April 15
- April 20
- April 26
- April 5
- April 6
- Armenian calendar
- Arthur Dobbs
- Assyrian calendar
- Atash Behram
- August 14
- August 18
- August 21
- August 26
- August 9
- Auto-da-fé
- Bahá'í calendar
- Beast of Gévaudan
- Bengali calendar
- Berber calendar
- Boston
- British Parliament
- Buddhist calendar
- Byzantine calendar
- Category 1765
- Category 1765 births
- Category 1765 deaths
- Category 1765 works
- Catherine the Great
- Charles Hatchett
- Chinese calendar
- Christian heresy
- Christianity
- Coptic calendar
- December 12
- December 16
- December 25
- December 3
- December 8
- Edward Young
- Eli Whitney
- Emma, Lady Hamilton
- Ethiopian calendar
- February 1
- George Glas
- Great Britain
- Gregorian calendar
- Hebrew calendar
- Henry Bouquet
- Hindu calendar
- Holocene calendar
- Holy Roman Emperor
- Horatio Nelson
- Iranian calendar
- Islamic calendar
- Isle of Man
- James Mackintosh
- James Watt
- January 11
- January 23
- Japanese calendar
- Jean Calas
- Jew
- John Adams
- Julian calendar
- July 14
- July 15
- July 26
- June 15
- June 21
- Kali Yuga
- Korean calendar
- Lisbon
- List of centuries
- List of decades
- List of years
- Luigi Schiavonetti
- March 22
- March 24
- March 27
- March 3
- March 7
- March 9
- Mary Bryant
- May 17
- May 18
- Meiwa
- Mikhail Lomonosov
- Millennium
- Minguo calendar
- Montreal
- Navsari
- Nicéphore Niépce
- North America
- November 1
- November 14
- November 17
- November 20
- November 30
- October 10
- October 17
- October 21
- October 24
- October 31
- October 8
- Paris
- Pope Gregory XVI
- Pyotr Bagration
- Quartering Act
- Quebec
- Regnal year
- Republic of China
- Restaurant
- Robert Fulton
- Roman numerals
- Russian Empire
- September 18
- September 2
- September 21
- September 6
- Sexagenary cycle
- Stamp Act 1765
- Steam engine
- Switzerland
- Tavern
- Thai solar calendar
- Thirteen Colonies
- Vaclav Prokop Divis
- Vikram Samvat
- Vodka
- Voltaire
- William Stukeley
- Abigail Adams
- Abigail Adams Smith
- Abigail Williams
- Andrew Oliver
- Antoine de Beauterne
- Arthur Dobbs
- Catherine the Great
- Charles Hatchett
- Edward Young
- Eli Whitney
- George Glas
- Henry Bouquet
- James Mackintosh
- James Watt
- Jean Calas
- John Adams
- Luigi Schiavonetti
- Mary Bryant
- Mikhail Lomonosov
- Nicéphore Niépce
- Pope Gregory XVI
- Pyotr Bagration
- Robert Fulton
- Voltaire
- William Stukeley
-

Stamp Act of 1765
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occurred in history! -

-

Report: Bahrain forces detained 1765 people in 2015
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom. According to the report, Bahraini security forces arrested one thousand seven hundred and sixty five people last year. The detainees included one hundred and twenty children. The report says there was a total ban on gathering and demonstrations across Bahrain in 2015. And the crackdown on protesters left over 700 people injured. The report was prepared by the Liberties and Human Rights Department of al-Wefaq National Islamic Society. Bahrain has been witnessing protests on a daily basis since the popular uprising began in the kingdom in February 2011. The crackdown on demonstrators has left many people dead. Colin Cavell Author and Lecturer Watch Live... -

-

Ocean Acidification 1765 - 2100
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in the ocean. For organisms with external calcium carbonate skeletons, such as corals and pteropods, the consequences can be very negative if the oceans become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. It has been demonstrated that pteropods, a planktonic mollusk, may not be able to maintain their shells in undersaturated waters. In some studies, live pteropods were subjected to waters that are undersaturated with respect to aragonite and their shells begin to dissolve within 48 hours (Feely et al., 2004; Orr et al 2005 ). Image of Ocean Acidification Color Bar This dataset has both historic ... -

1765 Glenridge Drive
For more details: http://view.paradym.com/3814731 1765 Glenridge Drive Kernersville, NC 27284 $268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299 Immaculate and open w/all the bells & whistles, lightly lived in. Elegant kitchen cabinets, granite countertops, hardwood floors. Generous master and suite, comfortable living area w/beautiful fireplace & gas logs, 2 bedrooms with a full bath, & a wonderful sunroom on 1st floor. The upper level offers a 4th bedroom & full bath. Tinted windows, ceiling fans throughout. Beautifully manicured lawn, concrete landscape border& decorative wrought iron fence surrounds the backyard. Presented By: Vicki Crutchfield, Coldwell Banker Triad, Realtors 336-362-9412 View My Inventory: http://my.paradym.com/inventory.asp?u=242251 -

-

-

RFX/ Algunos tienen poca memoria histórica (1765)
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica. -

RELAX... HAMPI 7 BLN... 0813 1765 7041
-

WIDO PKL... 3,1 KG.. 0813 1765 7041
-

1765 Gulf Blvd #202, Englewood, FL 34223
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 Other homes for sale by Joe Jenkins of (256010008) COLDWELL BANKER SUNSTAR REALTY: http://www.propertypanorama.com/all-tours/253242 Lowest Priced 3 Bedroom, 2 Bath Condo On Manasota Key, And It Is A Direct Bay Front Townhouse. Outstanding Full Bay Views From The Living & Dining Room Areas As Well As The Kitchen And Master Bedroom & There Are Even Partial Gulf Views From The 2Nd Bedroom. It Has A Good Rental History Of Over $21,000 Annually And That Is After The Owners Block It Out From Time To Time For Personal Use Or For Friends Or Other Family ...
Stamp Act of 1765
- Order: Reorder
- Duration: 12:40
- Updated: 07 Oct 2013
- views: 18097
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occu...
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occurred in history!
wn.com/Stamp Act Of 1765
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own any of this, this video complete belongs to PBS and all the events occurred in history!
- published: 07 Oct 2013
- views: 18097
(Arabian riding 1765) شرح عام و تركيب الشكيمة
- Order: Reorder
- Duration: 10:58
- Updated: 11 May 2016
- views: 57
Ahmad Alhuqayl / أحمد الحقيل
Google + https://plus.google.com/105644497358208945664/about
Twitter https://mobile.twitter.com/AAlhuqayl
Instagram https://instag...
Ahmad Alhuqayl / أحمد الحقيل
Google + https://plus.google.com/105644497358208945664/about
Twitter https://mobile.twitter.com/AAlhuqayl
Instagram https://instagram.com/ahmadalhuqayl/
My website http://ahmadalhuqayl.simdif.com
wn.com/(Arabian Riding 1765) شرح عام و تركيب الشكيمة
Report: Bahrain forces detained 1765 people in 2015
- Order: Reorder
- Duration: 5:26
- Updated: 11 May 2016
- views: 30
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom.
According to the report...
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom.
According to the report, Bahraini security forces arrested one thousand seven hundred and sixty five people last year. The detainees included one hundred and twenty children. The report says there was a total ban on gathering and demonstrations across Bahrain in 2015. And the crackdown on protesters left over 700 people injured. The report was prepared by the Liberties and Human Rights Department of al-Wefaq National Islamic Society. Bahrain has been witnessing protests on a daily basis since the popular uprising began in the kingdom in February 2011. The crackdown on demonstrators has left many people dead.
Colin Cavell
Author and Lecturer
Watch Live: http://www.presstv.ir/live.html
Twitter: http://twitter.com/PressTV
LiveLeak: http://www.liveleak.com/c/PressTV
Facebook: http://www.facebook.com/PRESSTV
Google+: http://plus.google.com/+VideosPTV
Instagram: http://instagram.com/presstvchannel
SoundCloud: https://soundcloud.com/videosptv
wn.com/Report Bahrain Forces Detained 1765 People In 2015
A new report shows the year 2015 was the worst for the Bahraini people regarding the human rights situation in the Persian Gulf kingdom.
According to the report, Bahraini security forces arrested one thousand seven hundred and sixty five people last year. The detainees included one hundred and twenty children. The report says there was a total ban on gathering and demonstrations across Bahrain in 2015. And the crackdown on protesters left over 700 people injured. The report was prepared by the Liberties and Human Rights Department of al-Wefaq National Islamic Society. Bahrain has been witnessing protests on a daily basis since the popular uprising began in the kingdom in February 2011. The crackdown on demonstrators has left many people dead.
Colin Cavell
Author and Lecturer
Watch Live: http://www.presstv.ir/live.html
Twitter: http://twitter.com/PressTV
LiveLeak: http://www.liveleak.com/c/PressTV
Facebook: http://www.facebook.com/PRESSTV
Google+: http://plus.google.com/+VideosPTV
Instagram: http://instagram.com/presstvchannel
SoundCloud: https://soundcloud.com/videosptv
- published: 11 May 2016
- views: 30
LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL.
- Order: Reorder
- Duration: 57:45
- Updated: 28 Aug 2015
- views: 511
Temas de consulta la sentencia C-358 de 1997, Dr. EDUARDO CIFUENTES MUÑOZ, SU- 1184 de 201, DR. EDUARDO MONTEALEGRE LYNET y C- 538 de 2008, DRA CLARA INES VARGA...
Temas de consulta la sentencia C-358 de 1997, Dr. EDUARDO CIFUENTES MUÑOZ, SU- 1184 de 201, DR. EDUARDO MONTEALEGRE LYNET y C- 538 de 2008, DRA CLARA INES VARGAS, sobre la competencia del Fuero penal Militar.
wn.com/Ley 1765 De 2015. Reestructura De La Justicia Penal Militar Y Policial.
Ocean Acidification 1765 - 2100
- Order: Reorder
- Duration: 0:34
- Updated: 17 Dec 2008
- views: 9377
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in t...
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in the ocean. For organisms with external calcium carbonate skeletons, such as corals and pteropods, the consequences can be very negative if the oceans become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. It has been demonstrated that pteropods, a planktonic mollusk, may not be able to maintain their shells in undersaturated waters. In some studies, live pteropods were subjected to waters that are undersaturated with respect to aragonite and their shells begin to dissolve within 48 hours (Feely et al., 2004; Orr et al 2005 ).
Image of Ocean Acidification Color Bar
This dataset has both historic and forecasted model-based changes in the ocean's carbonate chemistry due to increasing CO2 levels, as well as the presence of coral reefs depicted. The coral reefs are represented by black X's for shallow water species and magenta X's for deep water species. The ocean acidification levels are based on the median model output of the 13 international ocean carbon models based on the OCMIP-2 Project (Orr et al. 2005). The model outputs are represented on the global map in 5-yr increments from 1765 thru 2100. The future changes in CO2 chemistry were determined based on atmospheric CO2 scenarios presented in The Intergovernmental Panel on Climate Change (IPCC) IS92a 'continually increasing' CO2 emissions scenario (788 p.p.m.v. in the year 2100). As atmospheric levels of CO2 increase, the amount of CO2 that is absorbed by the ocean increases as well. This increase of CO2 in the ocean upsets the carbonate chemistry of the ocean and causes the waters to become more corrosive. The images indicate that all of the Southern Ocean surface waters south of 60°S and portions of the North Pacific become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. The scale to the left is proportional to the level of aragonite present in the ocean.
wn.com/Ocean Acidification 1765 2100
Monitoring ocean acidification is important because changes in the ocean's carbonate chemistry can have a major impact on the plant and animal life present in the ocean. For organisms with external calcium carbonate skeletons, such as corals and pteropods, the consequences can be very negative if the oceans become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. It has been demonstrated that pteropods, a planktonic mollusk, may not be able to maintain their shells in undersaturated waters. In some studies, live pteropods were subjected to waters that are undersaturated with respect to aragonite and their shells begin to dissolve within 48 hours (Feely et al., 2004; Orr et al 2005 ).
Image of Ocean Acidification Color Bar
This dataset has both historic and forecasted model-based changes in the ocean's carbonate chemistry due to increasing CO2 levels, as well as the presence of coral reefs depicted. The coral reefs are represented by black X's for shallow water species and magenta X's for deep water species. The ocean acidification levels are based on the median model output of the 13 international ocean carbon models based on the OCMIP-2 Project (Orr et al. 2005). The model outputs are represented on the global map in 5-yr increments from 1765 thru 2100. The future changes in CO2 chemistry were determined based on atmospheric CO2 scenarios presented in The Intergovernmental Panel on Climate Change (IPCC) IS92a 'continually increasing' CO2 emissions scenario (788 p.p.m.v. in the year 2100). As atmospheric levels of CO2 increase, the amount of CO2 that is absorbed by the ocean increases as well. This increase of CO2 in the ocean upsets the carbonate chemistry of the ocean and causes the waters to become more corrosive. The images indicate that all of the Southern Ocean surface waters south of 60°S and portions of the North Pacific become undersaturated (corrosive) with respect to aragonite, a form of calcium carbonate. The scale to the left is proportional to the level of aragonite present in the ocean.
- published: 17 Dec 2008
- views: 9377
1765 Glenridge Drive
- Order: Reorder
- Duration: 3:40
- Updated: 11 May 2016
- views: 0
For more details:
http://view.paradym.com/3814731
1765 Glenridge Drive
Kernersville, NC 27284
$268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299
Immacula...
For more details:
http://view.paradym.com/3814731
1765 Glenridge Drive
Kernersville, NC 27284
$268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299
Immaculate and open w/all the bells & whistles, lightly lived in. Elegant kitchen cabinets, granite countertops, hardwood floors. Generous master and suite, comfortable living area w/beautiful fireplace & gas logs, 2 bedrooms with a full bath, & a wonderful sunroom on 1st floor. The upper level offers a 4th bedroom & full bath. Tinted windows, ceiling fans throughout. Beautifully manicured lawn, concrete landscape border& decorative wrought iron fence surrounds the backyard.
Presented By:
Vicki Crutchfield, Coldwell Banker Triad, Realtors
336-362-9412
View My Inventory:
http://my.paradym.com/inventory.asp?u=242251
wn.com/1765 Glenridge Drive
For more details:
http://view.paradym.com/3814731
1765 Glenridge Drive
Kernersville, NC 27284
$268,900, 4 bed, 3.0 bath, 2,484 SF, MLS# 794299
Immaculate and open w/all the bells & whistles, lightly lived in. Elegant kitchen cabinets, granite countertops, hardwood floors. Generous master and suite, comfortable living area w/beautiful fireplace & gas logs, 2 bedrooms with a full bath, & a wonderful sunroom on 1st floor. The upper level offers a 4th bedroom & full bath. Tinted windows, ceiling fans throughout. Beautifully manicured lawn, concrete landscape border& decorative wrought iron fence surrounds the backyard.
Presented By:
Vicki Crutchfield, Coldwell Banker Triad, Realtors
336-362-9412
View My Inventory:
http://my.paradym.com/inventory.asp?u=242251
- published: 11 May 2016
- views: 0
1765 Rosewood Ln Ishpeming, MI 49849
- Order: Reorder
- Duration: 0:25
- Updated: 11 May 2016
- views: 0
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www.remax.com/mls/2319-1094268
Published on: May 11, 2016
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www.remax.com/mls/2319-1094268
Published on: May 11, 2016
wn.com/1765 Rosewood Ln Ishpeming, Mi 49849
1765 Rosewood Ln Ishpeming, MI 49849
- Order: Reorder
- Duration: 0:18
- Updated: 10 May 2016
- views: 0
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www.remax.com/mls/2319-1094268
Published on: May 10, 2016
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www.remax.com/mls/2319-1094268
Published on: May 10, 2016
wn.com/1765 Rosewood Ln Ishpeming, Mi 49849
RFX/ Algunos tienen poca memoria histórica (1765)
- Order: Reorder
- Duration: 5:30
- Updated: 20 Aug 2008
- views: 256265
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica.
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica.
wn.com/Rfx Algunos Tienen Poca Memoria Histórica (1765)
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca memoria histórica.
- published: 20 Aug 2008
- views: 256265
RELAX... HAMPI 7 BLN... 0813 1765 7041
- Order: Reorder
- Duration: 1:35
- Updated: 11 May 2016
- views: 21
- published: 11 May 2016
- views: 21
WIDO PKL... 3,1 KG.. 0813 1765 7041
- Order: Reorder
- Duration: 1:54
- Updated: 11 May 2016
- views: 21
- published: 11 May 2016
- views: 21
1765 Gulf Blvd #202, Englewood, FL 34223
- Order: Reorder
- Duration: 1:59
- Updated: 09 May 2016
- views: 1
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 ...
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 Other homes for sale by Joe Jenkins of (256010008) COLDWELL BANKER SUNSTAR REALTY: http://www.propertypanorama.com/all-tours/253242 Lowest Priced 3 Bedroom, 2 Bath Condo On Manasota Key, And It Is A Direct Bay Front Townhouse. Outstanding Full Bay Views From The Living & Dining Room Areas As Well As The Kitchen And Master Bedroom & There Are Even Partial Gulf Views From The 2Nd Bedroom. It Has A Good Rental History Of Over $21,000 Annually And That Is After The Owners Block It Out From Time To Time For Personal Use Or For Friends Or Other Family ...
wn.com/1765 Gulf Blvd 202, Englewood, Fl 34223
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for $364,900: http://www.propertypanorama.com/instaview-tour/mfr/D5906408 Other homes for sale by Joe Jenkins of (256010008) COLDWELL BANKER SUNSTAR REALTY: http://www.propertypanorama.com/all-tours/253242 Lowest Priced 3 Bedroom, 2 Bath Condo On Manasota Key, And It Is A Direct Bay Front Townhouse. Outstanding Full Bay Views From The Living & Dining Room Areas As Well As The Kitchen And Master Bedroom & There Are Even Partial Gulf Views From The 2Nd Bedroom. It Has A Good Rental History Of Over $21,000 Annually And That Is After The Owners Block It Out From Time To Time For Personal Use Or For Friends Or Other Family ...
- published: 09 May 2016
- views: 1
close fullscreen
- Playlist
- Chat
close fullscreen
- Playlist
- Chat
12:40

Stamp Act of 1765
This is the complete video of PBS's Stamp Act of 1765 video. Please enjoy, I do not own a...
published: 07 Oct 2013
Stamp Act of 1765
Stamp Act of 1765
- Report rights infringement
- published: 07 Oct 2013
- views: 18097
10:58

(Arabian riding 1765) شرح عام و تركيب الشكيمة
Ahmad Alhuqayl / أحمد الحقيل
Google + https://plus.google.com/105644497358208945664/about...
published: 11 May 2016
(Arabian riding 1765) شرح عام و تركيب الشكيمة
(Arabian riding 1765) شرح عام و تركيب الشكيمة
- Report rights infringement
- published: 11 May 2016
- views: 57
5:26

Report: Bahrain forces detained 1765 people in 2015
A new report shows the year 2015 was the worst for the Bahraini people regarding the human...
published: 11 May 2016
Report: Bahrain forces detained 1765 people in 2015
Report: Bahrain forces detained 1765 people in 2015
- Report rights infringement
- published: 11 May 2016
- views: 30
57:45

LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL.
Temas de consulta la sentencia C-358 de 1997, Dr. EDUARDO CIFUENTES MUÑOZ, SU- 1184 de 201...
published: 28 Aug 2015
LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL.
LEY 1765 de 2015. REESTRUCTURA DE LA JUSTICIA PENAL MILITAR Y POLICIAL.
- Report rights infringement
- published: 28 Aug 2015
- views: 511
0:34

Ocean Acidification 1765 - 2100
Monitoring ocean acidification is important because changes in the ocean's carbonate chemi...
published: 17 Dec 2008
Ocean Acidification 1765 - 2100
Ocean Acidification 1765 - 2100
- Report rights infringement
- published: 17 Dec 2008
- views: 9377
3:40

1765 Glenridge Drive
For more details:
http://view.paradym.com/3814731
1765 Glenridge Drive
Kernersville, ...
published: 11 May 2016
1765 Glenridge Drive
1765 Glenridge Drive
- Report rights infringement
- published: 11 May 2016
- views: 0
0:25

1765 Rosewood Ln Ishpeming, MI 49849
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www....
published: 11 May 2016
1765 Rosewood Ln Ishpeming, MI 49849
1765 Rosewood Ln Ishpeming, MI 49849
- Report rights infringement
- published: 11 May 2016
- views: 0
0:18

1765 Rosewood Ln Ishpeming, MI 49849
Crystal Barr Berglund - RE/MAX 1st Realty
3 beds 1 baths
For more information: http://www....
published: 10 May 2016
1765 Rosewood Ln Ishpeming, MI 49849
1765 Rosewood Ln Ishpeming, MI 49849
- Report rights infringement
- published: 10 May 2016
- views: 0
5:30

RFX/ Algunos tienen poca memoria histórica (1765)
Para los Anglosajones es muy fácil dar lecciones de humanidad al mundo; deben tener poca m...
published: 20 Aug 2008
RFX/ Algunos tienen poca memoria histórica (1765)
RFX/ Algunos tienen poca memoria histórica (1765)
- Report rights infringement
- published: 20 Aug 2008
- views: 256265
1:35

RELAX... HAMPI 7 BLN... 0813 1765 7041
published: 11 May 2016
RELAX... HAMPI 7 BLN... 0813 1765 7041
RELAX... HAMPI 7 BLN... 0813 1765 7041
- Report rights infringement
- published: 11 May 2016
- views: 21
1:54

WIDO PKL... 3,1 KG.. 0813 1765 7041
published: 11 May 2016
WIDO PKL... 3,1 KG.. 0813 1765 7041
WIDO PKL... 3,1 KG.. 0813 1765 7041
- Report rights infringement
- published: 11 May 2016
- views: 21
1:59

1765 Gulf Blvd #202, Englewood, FL 34223
The Virtual Tour for the property at 1765 Gulf Blvd #202, Englewood, FL 34223 selling for ...
published: 09 May 2016
1765 Gulf Blvd #202, Englewood, FL 34223
1765 Gulf Blvd #202, Englewood, FL 34223
- Report rights infringement
- published: 09 May 2016
- views: 1
Surplus Killings: When Wolves Behave Like Humans
Edit WorldNews.com 11 May 2016
Wasteful killings over time can in reality cause starvation among an entire pack. Scientists Puzzled Over Rare Surplus KillingTherefore, scientists were puzzled when a pack of gray wolves in northwestern Wyoming committed a rare occurrence ... Officials were also concerned the killings, two adult cows and nine calves, would cause unfounded fears among ranchers and hunters ... Animal nature is seldom wasteful ... It is displaced ... March, 2016. ....
Report: ISIS Android Alphabet App Appears Harmless, But Has Hidden Dangers
Edit WorldNews.com 11 May 2016
According to the jihadi monitoring site Vocativ, the app cannot be downloaded in the Google Store but only using AKP files that are being shared among the group’s supporters.ISIS has previously released apps, such as its “jihadist news feed” in November 2015, and a radio app in February that broadcasts the group’s propaganda....
Trump: Budweiser So Impressed What Country Will Become Changed Name To America
Edit WorldNews.com 11 May 2016
“I think so, they’re so impressed with what our country will become that they decided to do this before the fact.” – Republican presidential candidate Donald Trump. Anheuser-Busch announced the Budweiser name change and rebranding on Tuesday, saying it was repackaging 12-ounce bottles and cans with “America” from May 23 through the November election....
Queen says Chinese officials were 'very rude' during Xi Jinping's state visit
Edit The Guardian 11 May 2016
‘Oh, bad luck,’ Queen tells police commander in charge of visit security, in off-guard moment caught on camera at Buckingham Palace garden party. The “golden era” of UK-China relations appears to have lost some of its glitter after the Queen accused Chinese officials of being “very rude” to the British ambassador during president Xi Jinping’s first state visit to Britain last year ... “Oh, bad luck.”. Related ... Twitter ... Related ... Transcript ... ....
« back to news headlines
Rediscovering Jakuchu’s solitary pursuit of mastery
Edit The Japan News 10 May 2016
Ito Jakuchu's “Peonies and Birds” (before 1765) from his “Colorful Realm of Living Beings” series ... Ito Jakuchu's “Cranes and Plum Blossoms” (before 1765) from his “Colorful Realm of Living Beings” series....
MAM Software Group Schedules Conference Call for Tuesday May 17, 2016 10 May 2016 (MAM Software Group Inc)
Edit Public Technologies 10 May 2016
(Source. MAM Software Group Inc). BARNSLEY, England, May 10, 2016 /PRNewswire/ -- MAM Software Group, Inc. (NASDAQ Capital Market ... The Company has scheduled a conference call for Tuesday, May 17, 2016, at 9 a.m. ET to review the results. United States ... 719-785-1765 U.K.toll free ... (noodl. 33477941) ....
Through The Looking Glass: How Children's Books Have Grown Up
Edit National Public Radio 10 May 2016
i. Kindergarten students read before class starts at Walker-Jones Education Campus in Washington, D.C. Elissa Nadworny/NPR hide caption. toggle caption Elissa Nadworny/NPR. Kindergarten students read before class starts at Walker-Jones Education Campus in Washington, D.C. Elissa Nadworny/NPR. In Sarah Parrish's second-grade classroom, the colors are loud, but the kids are quiet ... Jones ... Take The History of Little Goody Two Shoes, from 1765 ... i....
EV Energy Partners Announces First Quarter 2016 Results (EV Energy Partners LP)
Edit Public Technologies 10 May 2016
(Source. EV Energy Partners LP) b6c8747f-3580-4a71-8ea0-cce5ce0251fa.pdf. May 10, 2016 EV Energy Partners Announces First Quarter 2016 Results ... NYMEX NYMEX ... WTI ... Investors interested in participating in the call may dial 1-888-510-1765 (quote conference ID 4054817) at least 5 minutes prior to the start time, or may listen live over the Internet through the Investor Relations section of the EVEP website at http.//www.evenergypartners.com....
QuinStreet Reports Financial Results for Third Quarter Fiscal Year 2016 (QuinStreet Inc)
Edit Public Technologies 10 May 2016
(Source. QuinStreet Inc). May 10, 2016. FOSTER CITY, Calif., May 10, 2016 (GLOBE NEWSWIRE) -- QuinStreet, Inc. (Nasdaq.QNST), the leader in performance marketing products and technologies, today announced financial results for the third quarter ended March 31, 2016 ... Adjusted EBITDA for the quarter was $3.5 million, or 4% of revenue ... Conference Call Today at 1.30 p.m. PT ... To access the conference call, dial (888) 510-1765 for the U.S ... Assets....
On this day: Great Train Robber Ronnie Biggs returns to Britain
Edit Scotsman 07 May 2016
Events, birthdays and anniversaries on 7 May. 1544. Earl of Hertford invaded Scotland in an attempt to force the Scottish estates to agree to the marriage of Edward, son of Henry VIII, and Mary Queen of Scots. Known as “The Rough Wooing”, it saw the burning and destruction of Border towns and abbeys and of Edinburgh. 1765. The warship Victory was launched at Chatham. She is now preserved at Portsmouth. 1791 ... 1824 ... 1832 ... 1848 ... 1888 ... 1907 ... 1915 ... ....
Cvent Announces First Quarter 2016 Financial Results
Edit Stockhouse 05 May 2016
... with the transaction (when they become available), and any other documents filed by Cvent with the SEC, may be obtained free of charge at the SEC’s website (http.//www.sec.gov) or at Cvent’s website (http.//investors.cvent.com) or by writing to Cvent’s Investor Relations at 1765 Greensboro Station Place, 7th Floor, Tysons Corner, Virginia 22102....
Cvent Announces First Quarter 2016 Financial Results (Cvent Inc)
Edit Public Technologies 05 May 2016
... with the transaction (when they become available), and any other documents filed by Cvent with the SEC, may be obtained free of charge at the SEC's website (http.//www.sec.gov) or at Cvent's website (http.//investors.cvent.com) or by writing to Cvent's Investor Relations at 1765 Greensboro Station Place, 7th Floor, Tysons Corner, Virginia 22102....
Grantown on Spey to mark its linen-making roots
Edit BBC News 04 May 2016
The footwear will then by hung on washing lines in the town ... Celebrations were held last summer to mark the laying of the first foundation stones of Grantown on Spey in 1765 ... ....
Ophthotech Reports First Quarter 2016 Financial and Operating Results
Edit Stockhouse 04 May 2016
Ophthotech Reports First Quarter 2016 Financial and Operating Results. – Conference Call and Webcast Today, May 4th, at 8.00 a.m. ET – ... “During the first quarter, we continued to prepare for the exciting opportunities that lie ahead in 2016,” said David R ... Recently, we had the privilege of welcoming Dr ... In April, Carmen A ... Dr ... To participate in this conference call, dial 888-359-3624 (USA) or 719-785-1765 (International), passcode 2126947....
Ophthotech Reports First Quarter 2016 Financial and Operating Results (Ophthotech Corporation)
Edit Public Technologies 04 May 2016
(Source. Ophthotech Corporation). May 4, 2016. - Conference Call and Webcast Today, May 4, at 8.00 a.m. ET - ... 'During the first quarter, we continued to prepare for the exciting opportunities that lie ahead in 2016,' said David R. Guyer, M.D., Chief Executive Officer and Chairman of the Board of Ophthotech ... In April, Carmen A ... To participate in this conference call, dial 888-359-3624 (USA) or 719-785-1765 (International), passcode 2126947....
- 1
- 2
- 3
- 4
- 5
- Next page »







