- published: 04 May 2014
- views: 22330
-
remove the playlistAtomic Nucleus
- remove the playlistAtomic Nucleus
- published: 10 Sep 2010
- views: 10019
- published: 27 Mar 2013
- views: 653
- published: 05 Feb 2013
- views: 206
- published: 27 Jul 2009
- views: 46793
- published: 12 Feb 2013
- views: 1990036
- published: 01 Mar 2008
- views: 273684
- published: 01 Oct 2012
- views: 69707
- published: 10 Oct 2008
- views: 27167
- published: 16 Apr 2012
- views: 1768950

The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932.[citation needed] Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the orbiting electrons.
The diameter of the nucleus is in the range of 1.75 fm (femtometre) (1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS (30 August 1871 – 19 October 1937) was a New Zealand chemist and physicist who became known as the father of nuclear physics. In early work he discovered the concept of radioactive half-life, proved that radioactivity involved the transmutation of one chemical element to another, and also differentiated and named alpha and beta radiation, proving that the former was essentially helium ions. This work was done at McGill University in Canada. It is the basis for the Nobel Prize in Chemistry he was awarded in 1908 "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances".
Rutherford performed his most famous work after he had moved to the Victoria University of Manchester in the UK in 1907 and was already a Nobel laureate. In 1911, he theorized that atoms have their positive charge concentrated in a very small nucleus, and thereby pioneered the Rutherford model of the atom, through his discovery and interpretation of Rutherford scattering in his gold foil experiment. He is widely credited with first "splitting the atom" in 1917 in a nuclear reaction between nitrogen and alpha particles, in which he also discovered (and named) the proton. This led to the first experiment to split the nucleus in a fully controlled manner, performed by two students working under his direction, John Cockcroft and Ernest Walton, in 1932. After his death in 1937, he was honoured by being interred with the greatest scientists of the United Kingdom, near Sir Isaac Newton's tomb in Westminster Abbey. The chemical element rutherfordium (element 104) was named after him in 1997.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
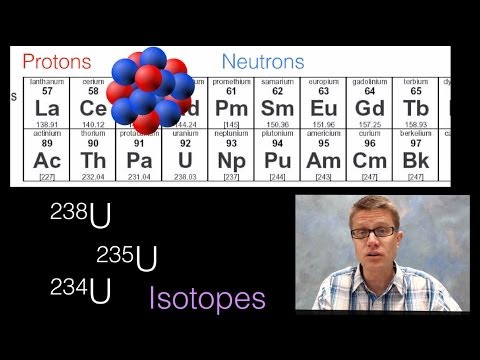 7:04
7:04Atomic Nucleus
Atomic NucleusAtomic Nucleus
003 - Atomic Nucleus In this video Paul Andersen explains how the structure of the nucleus influences the properties of the atom. The number of the protons determines the kind of element. Isotopes are formed when the number of protons remain the same but the neutrons are different. Some isotopes are radioactive and may decay over time. The rate of decay is the half-life and can be used to measure decay or time. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2008, Drawn by User:Fastfission in Illustrator and Inkscape--Fastfission 15:04, 14 April. English: Top: Expected Results of Rutherford's Gold Foil Experiment: Alpha Particles Passing through the Plum Pudding Model of the Atom Undisturbed. Bottom: Observed Results: Some of the Particles Were Deflected, and Some by Very Large Angles. Rutherford Concluded That the Positive Charge of the Atom Must Be Concentrated into a Very Small Location: The Atomic Nucleus., April 2008. Own work. http://commons.wikimedia.org/wiki/File:Rutherford_gold_foil_experiment_results.svg. "File:Alfa Beta Gamma Radiation.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Alfa_beta_gamma_radiation.svg. "File:Alpha Decay.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Alpha_Decay.svg. "File:Ernest Rutherford Cropped.jpg." Wikipedia, the Free Encyclopedia. Accessed April 26, 2014. http://en.wikipedia.org/wiki/File:Ernest_Rutherford_cropped.jpg. "File:Nucleus Drawing.svg." Wikipedia, the Free Encyclopedia. Accessed April 26, 2014. http://en.wikipedia.org/wiki/File:Nucleus_drawing.svg. File:Plum Pudding Atom.svg, n.d. http://commons.wikimedia.org/wiki/File:Plum_pudding_atom.svg. "File:Table Isotopes En.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Table_isotopes_en.svg. LeVanHan. English: Periodic Table of the Elements, 2008. Own work. http://commons.wikimedia.org/wiki/File:Periodic-table.jpg. London, Jay Springett from. English: Christmas Pudding with Flaming Rum, December 1, 2011. rum This photograph was submitted to Wikimedia Commons by "Will Murray (Willscrlt)". http://commons.wikimedia.org/wiki/File:Christmas_Pudding_with_Flaming_Rum.jpg. Rosenkrantz, Kurt. English: Decay of an Imaginary Radioactive Substance with a Half-Life of One Year., July 27, 2010. http://cafreetextbooks.ck12.org/science/CK12_Earth_Science_rev.pdf (page 433) If the above link no longer works, visit http://www.ck12.org and search for CK-12 Earth Science. http://commons.wikimedia.org/wiki/File:Radioactive_decay.png. -
 8:54
8:54Atomic Nucleus
Atomic NucleusAtomic Nucleus
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -
 1:06
1:06Atomic Nucleus
Atomic NucleusAtomic Nucleus
Check out us at:http://www.tutorvista.com/content/physics/physics-iv/atoms-and-nuclei/atomic-nucleus.php Atomic Nucleus he history of atomic models started way back in 400 B.C.A Greek scientist by name Democritus was the first to propose that the matter is composed of indivisible particles called Atoms.Atom in Greek means uncuttable.later Aristotle proposed that matter is composed of four elements earth,air,fire,water.this theory was wrong but it persisted for about 2000 years. Please like our facebook page http://www.facebook.com/tutorvista Follow us at: https://plus.google.com/+tutorvista -
 2:44
2:44ATOMIC NUCLEUS
ATOMIC NUCLEUSATOMIC NUCLEUS
We are similar in nature of the conglomerate aspect of procreation.. our soul's and body's are charged with energy from the sun.. if we have no atomic cause for being alive .. our life would cease to exist. Our atom's are made up of memory's,idea's, imagination and stardust .. as this is just a dream... " BatKlown " -
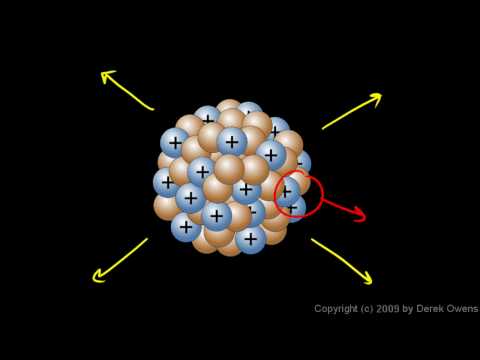 6:27
6:27Physical Science 7.4c - The Atomic Nucleus
Physical Science 7.4c - The Atomic NucleusPhysical Science 7.4c - The Atomic Nucleus
From the Physical Science course by Derek Owens. Eighth grade level. Distance Learning courses are available at http://www.derekowens.com -
 10:12
10:12The Nucleus: Crash Course Chemistry #1
The Nucleus: Crash Course Chemistry #1The Nucleus: Crash Course Chemistry #1
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is. Like CrashCourse? http://www.facebook.com/YouTubeCrashCourse Follow CrashCourse! http://www.twitter.com/TheCrashCourse Tumbl CrashCourse. http://thecrashcourse.tumblr.com Table of Contents Einstein & Atoms 02:05 Composition of Atoms 03:18 Atomic Number 04:20 Isotopes 08:04 Relative Atomic Mass 07:26 Mass Number 07:44 Watch the SciShow episodes on the Strong Nuclear Force here: http://www.youtube.com/watch?v=Yv3EMq2Dgq8 and http://www.youtube.com/watch?v=BNDOSMqGLlg Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 3:28
3:28The Discovery of the Atomic Nucleus (3 of 15)
The Discovery of the Atomic Nucleus (3 of 15)The Discovery of the Atomic Nucleus (3 of 15)
Episode 3 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.com/STFC_Matters Facebook: https://facebook.com/SciTechFacCouncil LinkedIn: https://www.linkedin.com/company/stfc -
 4:58
4:5839 1 The Atomic Nucleus and Radioactivity
39 1 The Atomic Nucleus and Radioactivity39 1 The Atomic Nucleus and Radioactivity
-
 4:14
4:14Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus
Basic Parts of the Atom - Protons, Neutrons, Electrons, NucleusBasic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus
The Beautiful pictures of the atom in this video come from Jefferson Lab @ https://www.jlab.org/. They are here on youtube too @ http://www.youtube.com/user/JeffersonLab. What are the Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus - This video describes the basic parts of the atom, including the proton, neutron and electron. The electrons are found in the electron cloud. This area takes up most of the space of the atom. The small and dense nucleus hold the protons and the neutrons. The protons and neutrons are measured by amu's, atomic mass units. -
 6:27
6:27Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)
Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)
Rutherfords Gold Foil -
 1:36
1:36Atomic nucleus - motivational song, Rate My Science
Atomic nucleus - motivational song, Rate My ScienceAtomic nucleus - motivational song, Rate My Science
http://ratemyscience.com/ Publish and rate science The nucleus of an atom is the very dense region, consisting of nucleons (protons and neutrons), at the center of an atom. Although the size of the nucleus varies considerably according to the mass of the atom, the size of the entire atom is comparatively constant. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, such as uranium). These dimensions are much smaller than the size of the atom itself by a factor of about 23,000 (uranium) to about 145,000 (hydrogen. -
 5:28
5:28Just How Small is an Atom?
Just How Small is an Atom?Just How Small is an Atom?
Just how small are atoms? And what's inside them? The answers turn out to be astounding, even for those who think they know. This fast-paced animation uses spectacular metaphors (imagine a blueberry the size of a football stadium!) to give a visceral sense of the building blocks that make our world. Lesson by Jonathan Bergmann, animation by Cognitive Media. -
 15:59
15:59Discovery of the Nucleus: Rutherford's Gold Foil Experiment
Discovery of the Nucleus: Rutherford's Gold Foil ExperimentDiscovery of the Nucleus: Rutherford's Gold Foil Experiment
To see all my Chemistry videos, check out http://socratic.org/chemistry In 1911, Ernest Rutherford and his colleagues discovered the nucleus of the atom using their famous gold foil experiment. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. This showed that atoms have a nucleus, and it disproved Thompson's plum pudding model of the atom. -
 5:18
5:18Calculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism Physics
Calculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism PhysicsCalculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism Physics
JJtheTutor.com How much work is needed to assemble an atomic nucleus containing three protons (such as Be) if we model it as an equilateral triangle of side with a proton at each vertex? Assume the protons started from very far away. Electric Potential, gauss's law, Coulombs, change in potential energy Vector addition in electric fields. Physics II 2, Calculus based physics. Electric flux, electric force dipole moment.
- Activation product
- Atom
- Atomic mass
- Atomic nucleus
- Atomic number
- Atomic orbital
- Atomic physics
- Axino
- Axion
- B meson
- Baryon
- Binding energy
- Bismuth-209
- Book Hadronic Matter
- Book Leptons
- Book Quarks
- Boron-14
- Borromean rings
- Boson
- Bottom antiquark
- Bottom quark
- Bound state
- Brachytherapy
- Bubble fusion
- CANDU reactor
- Chargino
- Charm antiquark
- Charm quark
- Chemical element
- D meson
- Davydov soliton
- Delta baryon
- Dense plasma focus
- Depleted uranium
- Deuterium
- Deuteron
- Dibaryon
- Dilaton
- Dineutron
- Diquark
- Dmitry Ivanenko
- Down antiquark
- Down quark
- Dry cask storage
- Electron
- Electron cloud
- Electron hole
- Electron neutrino
- Elementary particle
- Enriched uranium
- Ernest Marsden
- Ernest Rutherford
- Eta meson
- Eta prime meson
- Exciton
- Exotic atom
- Exotic baryon
- Exotic hadron
- Exotic meson
- Faddeev–Popov ghost
- Fast breeder reactor
- Fast neutron
- Fast-neutron reactor
- Femtometre
- Fermion
- Fertile material
- Fissile
- FLiBe
- Fusion neutron
- Fusion power
- Fusor
- Gamma camera
- Gauge boson
- Gaugino
- Giant resonance
- Gilbert N. Lewis
- Glueball
- Gluino
- Gluon
- Gravitino
- Graviton
- Hadron
- Halo nucleus
- Hans Geiger
- Heavy water reactor
- Helium
- Helium-3
- Helium-4
- Higgs boson
- Higgsino
- High level waste
- Hydrogen
- Hydrogen-2
- Hydrogen-3
- Hypernucleus
- Hyperon
- Ionizing radiation
- Isospin
- Isotope
- Isotope separation
- J ψ meson
- John Wiley & Sons
- Kaon
- Kluwer Academic
- Lambda baryon
- Lead-208
- Lepton
- Levitated dipole
- Light water reactor
- Liquid drop model
- Liquid helium
- Liquid-drop model
- List of baryons
- List of mesons
- List of particles
- Lithium-11
- Lithium-6
- Low level waste
- Magnetic monopole
- Magnon
- Magnox
- Majorana fermion
- Majoron
- Mass number
- Medical imaging
- Meson
- Mesonic molecule
- Metre
- Michael Faraday
- Migma
- Minor actinide
- Molecule
- Molten salt reactor
- Muon
- Muon neutrino
- Muonium
- Neutralino
- Neutron
- Neutron activation
- Neutron capture
- Neutron generator
- Neutron moderator
- Neutron poison
- Neutron radiation
- Neutron reflector
- Neutron temperature
- Nuclear arms race
- Nuclear chemistry
- Nuclear engineering
- Nuclear explosion
- Nuclear fission
- Nuclear force
- Nuclear fuel
- Nuclear fuel cycle
- Nuclear fusion
- Nuclear medicine
- Nuclear physics
- Nuclear power
- Nuclear power debate
- Nuclear power plant
- Nuclear propulsion
- Nuclear reactor
- Nuclear reprocessing
- Nuclear safety
- Nuclear shell model
- Nuclear size
- Nuclear structure
- Nuclear technology
- Nuclear warfare
- Nuclear weapon
- Nuclear weapon yield
- Nucleon
- Omega baryon
- Omega meson
- Onium
- Particle physics
- Pebble bed reactor
- Pentaquark
- Phi meson
- Phonon
- Photon
- Pion
- Plasmaron
- Plasmon
- Plum pudding model
- Plutonium
- Polariton
- Polaron
- Polywell
- Pomeron
- Positron
- Positronium
- Prolate
- Promethium
- Proton
- Proton therapy
- Pyroelectric fusion
- Quantum number
- Quark
- Quarkonium
- Quasiparticle
- Radiation
- Radiation therapy
- Radioactive waste
- Radioactivity
- RBMK
- Reprocessed uranium
- Reversed field pinch
- Rho meson
- Roton
- Rutherford model
- Schrödinger
- Scintigraphy
- Sfermion
- SGHWR
- Sigma baryon
- Skyrmion
- Spent fuel pool
- Spent nuclear fuel
- Spheromak
- Springer (publisher)
- SSTAR
- Stellarator
- Sterile neutrino
- Strange antiquark
- Strange quark
- Strangeness
- Strong interaction
- Superatom
- Superfluid
- Superpartner
- T meson
- Tachyon
- Talk Atomic nucleus
- Tau (particle)
- Tau neutrino
- Tauonium
- Technetium
- Template Particles
- Tetraquark
- Thermal neutron
- Theta meson
- Thorium
- Tin
- TNT equivalent
- Tokamak
- Tomotherapy
- Top antiquark
- Top quark
- Trion (physics)
- Tritium
- UHTREX
- Up antiquark
- Up quark
- Upsilon meson
- Uranium
- Van der Waals force
- VVER
- W and Z bosons
- W' and Z' bosons
- Wave function
- Wikipedia Books
- X and Y bosons
- X-ray
- Xi baryon
- Yukawa potential
- Z-pinch
-

Atomic Nucleus
003 - Atomic Nucleus In this video Paul Andersen explains how the structure of the nucleus influences the properties of the atom. The number of the protons determines the kind of element. Isotopes are formed when the number of protons remain the same but the neutrons are different. Some isotopes are radioactive and may decay over time. The rate of decay is the half-life and can be used to measure decay or time. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2008, Drawn by User:Fastfission in Illustrator and Inkscape--Fastfission 15:... -

Atomic Nucleus
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -

Atomic Nucleus
Check out us at:http://www.tutorvista.com/content/physics/physics-iv/atoms-and-nuclei/atomic-nucleus.php Atomic Nucleus he history of atomic models started way back in 400 B.C.A Greek scientist by name Democritus was the first to propose that the matter is composed of indivisible particles called Atoms.Atom in Greek means uncuttable.later Aristotle proposed that matter is composed of four elements earth,air,fire,water.this theory was wrong but it persisted for about 2000 years. Please like our facebook page http://www.facebook.com/tutorvista Follow us at: https://plus.google.com/+tutorvista -

ATOMIC NUCLEUS
We are similar in nature of the conglomerate aspect of procreation.. our soul's and body's are charged with energy from the sun.. if we have no atomic cause for being alive .. our life would cease to exist. Our atom's are made up of memory's,idea's, imagination and stardust .. as this is just a dream... " BatKlown " -

Physical Science 7.4c - The Atomic Nucleus
From the Physical Science course by Derek Owens. Eighth grade level. Distance Learning courses are available at http://www.derekowens.com -

The Nucleus: Crash Course Chemistry #1
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is. Like CrashCourse? http://www.facebook.com/YouTubeCrashCourse Follow CrashCourse! http://www.twitter.com/TheCrashCourse Tumbl CrashCourse. http://thecrashcourse.tumblr.com Table of Contents Einstein & Atoms 02:05 Composition of Atoms 03:18 Atomic Num... -

The Discovery of the Atomic Nucleus (3 of 15)
Episode 3 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.co... -

39 1 The Atomic Nucleus and Radioactivity
-

Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus
The Beautiful pictures of the atom in this video come from Jefferson Lab @ https://www.jlab.org/. They are here on youtube too @ http://www.youtube.com/user/JeffersonLab. What are the Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus - This video describes the basic parts of the atom, including the proton, neutron and electron. The electrons are found in the electron cloud. This area takes up most of the space of the atom. The small and dense nucleus hold the protons and the neutrons. The protons and neutrons are measured by amu's, atomic mass units. -

Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)
Rutherfords Gold Foil -

Atomic nucleus - motivational song, Rate My Science
http://ratemyscience.com/ Publish and rate science The nucleus of an atom is the very dense region, consisting of nucleons (protons and neutrons), at the center of an atom. Although the size of the nucleus varies considerably according to the mass of the atom, the size of the entire atom is comparatively constant. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, such as uranium). These dimensions are much smaller than the size of the atom itself by a factor of about 23,000 (uranium) to about 145,000 (hydrogen. -

Just How Small is an Atom?
Just how small are atoms? And what's inside them? The answers turn out to be astounding, even for those who think they know. This fast-paced animation uses spectacular metaphors (imagine a blueberry the size of a football stadium!) to give a visceral sense of the building blocks that make our world. Lesson by Jonathan Bergmann, animation by Cognitive Media. -

Discovery of the Nucleus: Rutherford's Gold Foil Experiment
To see all my Chemistry videos, check out http://socratic.org/chemistry In 1911, Ernest Rutherford and his colleagues discovered the nucleus of the atom using their famous gold foil experiment. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. This showed that atoms have a nucleus, and it disproved Thompson's plum pudding model of the atom. -

Calculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism Physics
JJtheTutor.com How much work is needed to assemble an atomic nucleus containing three protons (such as Be) if we model it as an equilateral triangle of side with a proton at each vertex? Assume the protons started from very far away. Electric Potential, gauss's law, Coulombs, change in potential energy Vector addition in electric fields. Physics II 2, Calculus based physics. Electric flux, electric force dipole moment. -

Fischer: What Keeps An Atom's Nucleus Together? Jesus
Bryan Fischer says that Jesus is the force that holds the atomic nucleus together. -

Discovery of the Atomic Nucleus: Gold Foil Experiment (Ernest Rutherford - 1911)
Don't forget to LIKE, COMMENT, and SUBSCRIBE: http://www.youtube.com/subscription_center?add_user=MoofUniversity SUPPORT MOOF UNIVERSITY: http://www.moofuniversity.com/support-moof/ BUY A T-SHIRT https://shop.spreadshirt.com/moofuniversity/ INFORMATION ABOUT TUTORING: http://www.moofuniversity.com/tutoring/ INSTAGRAM: https://instagram.com/moofuniversity/ FACEBOOK: https://www.facebook.com/pages/Moof-University/1554858934727545 TWITTER: https://twitter.com/moofuniversity -

Atomic Nucleus Cesium to Radon
Atomic Nucleus Cesium to Radon -

Amazing atomic nucleus animation nuclear emblem atomic icon HD animated cartoon
Amazing atomic nucleus animation nuclear emblem atomic icon HD animated cartoon -

Atomic Nucleus Francium to Ununoctium
Atomic Nucleus Francium to Ununoctium -

A Level Physics - The Size, Mass and Density of the Nucleus
Find out a little more about the masses of the subatomic particles and the Atomic Mass Unit, u. You can also estimate the size of the atomic nuclei and therefore calculate their density which is massive! If you would like to see more A Level Physics videos then please Subscribe to my channel to keep updated with new videos and to search the Playlists already created. You can also visit my site 'A Level Physics Online' to see how all the videos relate to your course and for even more resources at http://www.alevelphysicsonline.com/ Thanks for watching, Mr Matheson -

-

Chemistry Tutorial 3.01a: Atomic Structure - The Nucleus
This video outlines the particles found in the nucleus, mass number, atomic number, nuclear charge and isotopes. -

ntid_lexicon's sign for "Atomic Nucleus"
Courtesy of the NTID Science Signs Project at www.rit.edu/ntid/sciencesigns. Signs are continually improved with evaluation and feedback. Please see the NTID online lexicon for updates on this sign. This sign video was contributed by users of the ASL-STEM Forum: http://aslstem.cs.washington.edu/ This sign video was contributed by users of the ASL-STEM Forum: http://aslstem.cs.washington.edu/
Atomic Nucleus
- Order: Reorder
- Duration: 7:04
- Updated: 04 May 2014
- views: 22330
- published: 04 May 2014
- views: 22330
Atomic Nucleus
- Order: Reorder
- Duration: 8:54
- Updated: 10 Sep 2010
- views: 10019
- published: 10 Sep 2010
- views: 10019
Atomic Nucleus
- Order: Reorder
- Duration: 1:06
- Updated: 27 Mar 2013
- views: 653
- published: 27 Mar 2013
- views: 653
ATOMIC NUCLEUS
- Order: Reorder
- Duration: 2:44
- Updated: 05 Feb 2013
- views: 206
- published: 05 Feb 2013
- views: 206
Physical Science 7.4c - The Atomic Nucleus
- Order: Reorder
- Duration: 6:27
- Updated: 27 Jul 2009
- views: 46793
- published: 27 Jul 2009
- views: 46793
The Nucleus: Crash Course Chemistry #1
- Order: Reorder
- Duration: 10:12
- Updated: 12 Feb 2013
- views: 1990036
- published: 12 Feb 2013
- views: 1990036
The Discovery of the Atomic Nucleus (3 of 15)
- Order: Reorder
- Duration: 3:28
- Updated: 01 Mar 2008
- views: 273684
- published: 01 Mar 2008
- views: 273684
39 1 The Atomic Nucleus and Radioactivity
- Order: Reorder
- Duration: 4:58
- Updated: 20 Mar 2014
- views: 1576
- published: 20 Mar 2014
- views: 1576
Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus
- Order: Reorder
- Duration: 4:14
- Updated: 01 Oct 2012
- views: 69707
- published: 01 Oct 2012
- views: 69707
Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)
- Order: Reorder
- Duration: 6:27
- Updated: 01 Oct 2013
- views: 8380
Atomic nucleus - motivational song, Rate My Science
- Order: Reorder
- Duration: 1:36
- Updated: 10 Oct 2008
- views: 27167
- published: 10 Oct 2008
- views: 27167
Just How Small is an Atom?
- Order: Reorder
- Duration: 5:28
- Updated: 16 Apr 2012
- views: 1768950
- published: 16 Apr 2012
- views: 1768950
Discovery of the Nucleus: Rutherford's Gold Foil Experiment
- Order: Reorder
- Duration: 15:59
- Updated: 10 Nov 2012
- views: 103453
- published: 10 Nov 2012
- views: 103453
Calculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism Physics
- Order: Reorder
- Duration: 5:18
- Updated: 14 Feb 2013
- views: 1843
- published: 14 Feb 2013
- views: 1843
Fischer: What Keeps An Atom's Nucleus Together? Jesus
- Order: Reorder
- Duration: 1:12
- Updated: 05 Jun 2013
- views: 22770
- published: 05 Jun 2013
- views: 22770
Discovery of the Atomic Nucleus: Gold Foil Experiment (Ernest Rutherford - 1911)
- Order: Reorder
- Duration: 8:00
- Updated: 17 Aug 2015
- views: 225
- published: 17 Aug 2015
- views: 225
Atomic Nucleus Cesium to Radon
- Order: Reorder
- Duration: 13:31
- Updated: 31 Oct 2014
- views: 64
Amazing atomic nucleus animation nuclear emblem atomic icon HD animated cartoon
- Order: Reorder
- Duration: 1:01
- Updated: 29 Apr 2013
- views: 1861
- published: 29 Apr 2013
- views: 1861
Atomic Nucleus Francium to Ununoctium
- Order: Reorder
- Duration: 13:30
- Updated: 30 Oct 2014
- views: 467
A Level Physics - The Size, Mass and Density of the Nucleus
- Order: Reorder
- Duration: 4:18
- Updated: 02 Feb 2016
- views: 530
- published: 02 Feb 2016
- views: 530
Atomic Nucleus Rubidium to Xenon
- Order: Reorder
- Duration: 7:41
- Updated: 30 Oct 2014
- views: 91
Chemistry Tutorial 3.01a: Atomic Structure - The Nucleus
- Order: Reorder
- Duration: 8:54
- Updated: 11 Sep 2009
- views: 52359
- published: 11 Sep 2009
- views: 52359
ntid_lexicon's sign for "Atomic Nucleus"
- Order: Reorder
- Duration: 0:11
- Updated: 08 Aug 2011
- views: 125
- published: 08 Aug 2011
- views: 125
- Playlist
- Chat
- Playlist
- Chat

Atomic Nucleus
- Report rights infringement
- published: 04 May 2014
- views: 22330

Atomic Nucleus
- Report rights infringement
- published: 10 Sep 2010
- views: 10019

Atomic Nucleus
- Report rights infringement
- published: 27 Mar 2013
- views: 653

ATOMIC NUCLEUS
- Report rights infringement
- published: 05 Feb 2013
- views: 206

Physical Science 7.4c - The Atomic Nucleus
- Report rights infringement
- published: 27 Jul 2009
- views: 46793

The Nucleus: Crash Course Chemistry #1
- Report rights infringement
- published: 12 Feb 2013
- views: 1990036

The Discovery of the Atomic Nucleus (3 of 15)
- Report rights infringement
- published: 01 Mar 2008
- views: 273684

39 1 The Atomic Nucleus and Radioactivity
- Report rights infringement
- published: 20 Mar 2014
- views: 1576

Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus
- Report rights infringement
- published: 01 Oct 2012
- views: 69707

Rutherfords Gold Foil Experiment (Discovery of the Atomic Nucleus)
- Report rights infringement
- published: 01 Oct 2013
- views: 8380

Atomic nucleus - motivational song, Rate My Science
- Report rights infringement
- published: 10 Oct 2008
- views: 27167

Just How Small is an Atom?
- Report rights infringement
- published: 16 Apr 2012
- views: 1768950

Discovery of the Nucleus: Rutherford's Gold Foil Experiment
- Report rights infringement
- published: 10 Nov 2012
- views: 103453

Calculating Work Needed to Assemble an Atomic Nucleus - Electromagnetism Physics
- Report rights infringement
- published: 14 Feb 2013
- views: 1843
Wrestling star Chyna found dead at Redondo Beach home
Edit Orlando Sentinel 21 Apr 2016Madeleine Albright: Putin a 'Smart, But Truly Evil Man'
Edit WorldNews.com 20 Apr 2016Victoria Wood dead: Comedian dies aged 62 from cancer
Edit The Independent 20 Apr 2016Former Co-Workers Claim North Korean Defectors Were Abducted
Edit WorldNews.com 21 Apr 2016Queen becomes UK's first nonagenarian sovereign
Edit Belfast Telegraph 21 Apr 2016Global Radiopharmaceuticals Market Outlook 2020 - Key Players GE, Bayer HealthCare & Cardinal Health - ...
Edit Business Wire 20 Apr 2016'Cosmic Eye' goes viral (University of Western Australia)
Edit Public Technologies 18 Apr 2016Minister Goodale celebrates cutting-edge infrastructure for researchers (Department of Industry of Canada)
Edit Public Technologies 15 Apr 2016Minister Goodale celebrates cutting-edge infrastructure for researchers (Embassy of Canada in France)
Edit Public Technologies 15 Apr 2016Minister Goodale celebrates cutting-edge infrastructure for researchers (ITO - Industrial Technologies Office)
Edit Public Technologies 15 Apr 2016Minister Goodale celebrates cutting-edge infrastructure for researchers (Government of Canada)
Edit Public Technologies 15 Apr 2016Gonzaga Presents Faculty Neighborhood Cafes April 23 (Gonzaga University)
Edit Public Technologies 12 Apr 2016Rare events may be required to form the Universe’s heaviest metals [US]
Edit Ars Technica 05 Apr 2016Rare events may be required to form the Universe’s heaviest metals
Edit Ars Technica 05 Apr 2016Reactions: Stuffing the Periodic Table suitcase (Illinois State University)
Edit Public Technologies 25 Mar 2016High school physics students get a taste of particle astrophysics (University of Delaware)
Edit Public Technologies 17 Mar 2016Deep inside the gravitational wave phenomenon
Edit Deccan Herald 14 Mar 2016How Things Break (And Why Scientists Want to Know) (Argonne National Laboratory)
Edit Public Technologies 07 Mar 2016- 1
- 2
- 3
- 4
- 5
- Next page »







