- published: 03 Sep 2009
- views: 378750
-
remove the playlistSolubility
- remove the playlistSolubility
- published: 26 Feb 2014
- views: 52032
- published: 10 May 2012
- views: 61707
- published: 27 Jul 2012
- views: 198790
- published: 21 Sep 2014
- views: 34422
- published: 26 Mar 2013
- views: 598325
- published: 08 May 2012
- views: 12763
- published: 02 Apr 2015
- views: 2424
- published: 10 Jun 2011
- views: 68964
- published: 18 Mar 2014
- views: 35752

Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution.
Most often, the solvent is a liquid, which can be a pure substance or a mixture. One may also speak of solid solution, but rarely of solution in a gas (see vapor-liquid equilibrium instead).
The extent of solubility ranges widely, from infinitely soluble (fully miscible) such as ethanol in water, to poorly soluble, such as silver chloride in water. The term insoluble is often applied to poorly or very poorly soluble compounds.
Under certain conditions, the equilibrium solubility can be exceeded to give a so-called supersaturated solution, which is metastable.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 13:22
13:22Solubility and intermolecular forces | Chemistry | Khan Academy
Solubility and intermolecular forces | Chemistry | Khan AcademySolubility and intermolecular forces | Chemistry | Khan Academy
Solubility of salt and gas solutes in liquid solvent. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/introduction-to-intermolecular-forces/v/surface-tension?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/introduction-to-intermolecular-forces/v/van-der-waals-forces?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan AcademyâÂÂs Chemistry channel: https://www.youtube.com/channel/UCyEot66LrwWFEMONvrIBh3A?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
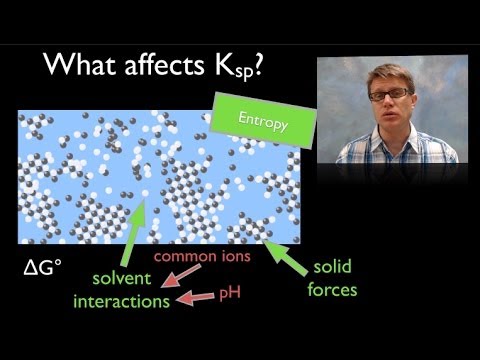 7:06
7:06Solubility
SolubilitySolubility
070 - Solubility In this video Paul Andersen explains how the dissolution of a solute in a solution can be explained as a reversible reaction. Bonds in the solid solute are broken and the ions are dissolved in a solution. The Ksp (or solubility product constant) can be used to explain the solubility of various salts. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2004, Picture taken by me-- Chris 73 14:12, 11 Dec. English: Solution of Salt in Water (regular Table Salt, Regular Tap water)Esperanto: Salo Solviĝanta En akvoPolski: Sól Rozpuszczana W wodzieРусский: Растворение Соли В Воде, [object HTMLTableCellElement]. Licensing: This image was created by Chris 73. The image is licensed under a dual license; please choose either of the two licenses below as desired. Attribution to Wikipedia or another project of the Wikimedia foundation is required for both licenses if the image is used outside of projects of the Wikimedia foundation. Attribution to me is not required. Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.3 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. A copy of the license is included in the section entitled GNU Free Documentation License. www.gnu.org/licenses/fdl-1.3.htmlGFDL 1.3GNU Free Documentation License 1.3truetrue This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. Attribution: Chris 73 / Wikimedia Commons You are free: to share -- to copy, distribute and transmit the work to remix -- to adapt the work Under the following conditions: attribution -- You must attribute the work in the manner specified by the author or licensor (but not in any way that suggests that they endorse you or your use of the work). share alike -- If you alter, transform, or build upon this work, you may distribute the resulting work only under the same or similar license to this one. http://creativecommons.org/licenses/by-sa/3.0 CC-BY-SA-3.0 Creative Commons Attribution-Share Alike 3.0 truetrue "I want to use the image. How do I do that?" You can use this image freely for any purpose, including commercial use, provided that you license it under one of the above licenses. My suggestion is to use the following text: This Wikipedia and Wikimedia Commons image is from the user Chris 73 and is freely available at //commons.wikimedia.org/wiki/File:SaltInWaterSolutionLiquid.jpg under the creative commons cc-by-sa 3.0 license. For privacy reasons please use only "Chris 73" as author. If necessary, please translate the text in your language. For electronic use please include the links in the text as shown, for printed use please print the text as shown. If you use the image I would appreciate it if you would let me know on my talk page, but this is not required as long as you follow one of the above licenses. http://commons.wikimedia.org/wiki/File:SaltInWaterSolutionLiquid.jpg. "File:Pollution - Damaged by Acid Rain.jpg." Wikipedia, the Free Encyclopedia. Accessed February 16, 2014. http://en.wikipedia.org/wiki/File:Pollution_-_Damaged_by_acid_rain.jpg. "File:Sea Salt-E-Dp Hg.svg." Wikipedia, the Free Encyclopedia. Accessed February 16, 2014. http://en.wikipedia.org/wiki/File:Sea_salt-e-dp_hg.svg. "Salts & Solubility." PhET. Accessed February 16, 2014. http://phet.colorado.edu/en/simulation/soluble-salts. -
 3:32
3:32solubility
solubilitysolubility
-
 14:15
14:15Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions
Solubility Curves - Saturated, Unsaturated, Supersaturated SolutionsSolubility Curves - Saturated, Unsaturated, Supersaturated Solutions
CLEAR & SIMPLE Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions - This video explains how to interpret solubility curves and read solubility curves. It discusses saturated, unsaturated and supersaturated solutions. This is an essential component of the unit on solutions/mixtures of substances. In a nutshell, unsaturated solutions have points below the line on the graph, saturated is on the line and supersaturated is above the line on the graph. -
 2:41
2:41Solubility Rules (Mnemonic Tricks)
Solubility Rules (Mnemonic Tricks)Solubility Rules (Mnemonic Tricks)
Annoyed by all those kids sagging their pants? Well, here's a good way to put that distrubance to use. (It shall help you with your solubility endeavors) -
 11:45
11:45Solubility product constant from the solubility
Solubility product constant from the solubilitySolubility product constant from the solubility
How to calculate the solubility (and the molar solubility) of a slightly soluble ionic compound and how to use the solubility to calculate the solubility product constant -
 13:34
13:34Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7
Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7
Dihydrogen monoxide (better know as water) is the key to nearly everything. It falls from the sky, makes up 60% of our bodies, and just about every chemical process related to life takes place with it or in it. Without it, none of the chemical reactions that keep us alive would happen - none of the reactions that sustain any life form on earth would happen - and the majority of inorganic chemical reactions that shape the surface of the earth would not happen either. Every one of us uses water for all kinds of chemistry every day - our body chemistry, our food chemistry and our laundry chemistry all take place in water. In today's Crash Course Chemistry, we use Hank's actual dirty laundry (ew) to learn about some of the properties of water that make it so special - it's polarity and dielectric property; how electrolytes can be used to classify solutions; and we discover how to calculate a solution's molarity as well as how to dilute a solution using the dilution equation. Table of Contents Polarity 02:40 Dielectric Property 04:13 Electrolytes 04:29 Molarity 08:46 Dilution 10:56 Want to find Crash Course elsewhere on the internet? Facebook - http://www.facebook.com/YouTubeCrashCourse Twitter - http://www.twitter.com/TheCrashCourse Tumblr - http://thecrashcourse.tumblr.com Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 9:09
9:09Factors that Affect Solubility - CLEAR & SIMPLE
Factors that Affect Solubility - CLEAR & SIMPLEFactors that Affect Solubility - CLEAR & SIMPLE
CLEAR & SIMPLE - Solubility of Substances - What Changes the Solubility of a Substance - Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions. This video explains the factors that affect solubility. The video explains the relationship between heat-temperature and solubility, pressure and solubility, surface area and solubility, agitation-shaking and solubility and the type of solvent, ionic, polar covalent, nonpolar covalent. -
 13:55
13:55Solubility Explained
Solubility ExplainedSolubility Explained
In this video I will explain the how and why different substances dissolve in water. I will also explain the polar nature of water -
 5:14
5:14Solubility of Ionic Compounds: Basics and Rules
Solubility of Ionic Compounds: Basics and RulesSolubility of Ionic Compounds: Basics and Rules
Basic discussion on the solubility of ionic compounds and the rules for determining whether an ionic compound is soluble or insoluble. -
 6:37
6:37Solubility Rules and Precipitation Reactions - Mr. Causey's Chemistry
Solubility Rules and Precipitation Reactions - Mr. Causey's ChemistrySolubility Rules and Precipitation Reactions - Mr. Causey's Chemistry
Mr. Causey discusses the solubility rules and the importance of knowing these rules in order to predict if a precipitate will form during a reaction. http://www.yourCHEMcoach.com Learn more and understand better with Mr. Causey's tutorials. Subscribe for more chemistry videos: http://bit.ly/1jeutVl Share this Video: https://www.youtube.com/watch?v=UbyqA5mi6GU Resources: Polyatomic Ion Cheat Sheet: http://bit.ly/14e2pbw Related Videos: Aqueous Solutions: http://www.youtube.com/watch?v=vg5FUNIrW3o Net Ionic Equations: http://www.youtube.com/watch?v=vbx5nfAiBlM Contact Me: mrcausey@mrcausey.com Follow Me: http://www.twitter.com/#!/mrcausey http://pinterest.com/mistercausey/ http://www.facebook.com/profile.php?id=814523544 In order to predict if a precipitate will form during a reaction, you need to use the solubility rules. These rules are also useful in writing net ionic equations. -
 1:22
1:22Solubility Song
Solubility SongSolubility Song
Solubility Rules in the form of a song -
 3:15
3:15The Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr Pauller
The Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr PaullerThe Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr Pauller
This video shows a demonstration which illustrates how the solubility of carbon dioxide in water changes due to changes in water temperature. -
 21:02
21:02Solutions Lesson 1 Solutions and Solubility
Solutions Lesson 1 Solutions and SolubilitySolutions Lesson 1 Solutions and Solubility
- Aerosinusitis
- Air embolism
- Albert A. Bühlmann
- Albert R. Behnke
- Albite
- Alkali metal
- Alkaline earth metal
- Alloy
- Alternobaric vertigo
- Ambient pressure
- Ammonium
- Amorphous
- Amphiphile
- Anhydrous
- Anti fog
- Aphorism
- Aqueous
- Aqueous solution
- Aragonite
- Arthur J. Bachrach
- Asphyxia
- Aspirin
- Atmosphere (unit)
- Atmospheric pressure
- Atrial septal defect
- Avascular necrosis
- Barium
- Barium nitrate
- Barodontalgia
- Barostriction
- Barotrauma
- Base metal
- Benzene
- Benzoic acid
- Blood shift
- Blood-air barrier
- Boyle's law
- Bromide
- Buffer solution
- Buoyancy
- Calcite
- Calcium
- Calcium carbonate
- Calcium sulfate
- Carbonate
- Category Solvents
- Celsius
- Cerium(III) sulfate
- Charles Momsen
- Charles' law
- Chem. Commun.
- Chemical formula
- Chemical kinetics
- Chemical polarity
- Chemical structure
- Chemical substance
- Chloride
- Cold shock response
- Colloid
- Combined gas law
- Common-ion effect
- Complex (chemistry)
- Concentration
- Copper
- Copper(II) sulfate
- CO₂ retention
- Critical temperature
- Crowdsourcing
- Cyclodextrin
- Dalton's law
- Deep water blackout
- Dental barotrauma
- Dichloromethane
- Dielectric constant
- Diethyl ether
- Diffusion
- Dilution (equation)
- Divers Alert Network
- Diving chamber
- Diving medicine
- Diving physics
- Drowning
- Drug delivery
- Dry ice
- Dysbarism
- Dühring's rule
- Ear clearing
- Edward D. Thalmann
- Electronegativity
- Enthalpy of fusion
- Enthalpy of solution
- Entropy
- Entropy of mixing
- Equilibrium constant
- Ethanol
- Ethyl alcohol
- Eutectic system
- Felix Hoppe-Seyler
- Filtration
- Force
- Fouling
- Frenzel maneuver
- Gas
- Gas exchange
- Gaseous
- Gasoline
- Gay-Lussac's law
- George F. Bond
- Gibbsite
- Henry's law
- Hot water extraction
- Hydrate
- Hydrogen narcosis
- Hydrophile
- Hydrophobe
- Hydrophobic effect
- Hydrostatic pressure
- Hydrotrope
- Hydroxide
- Hyperbaric medicine
- Hypercapnia
- Hyperoxia
- Hypothermia
- Hypoxia (medical)
- Ideal gas law
- Ideal solution
- Immersion diuresis
- Indigo dye
- Intermolecular force
- Iodide
- Ionic strength
- Iron(II) hydroxide
- IUPAC
- Jacques Triger
- John Scott Haldane
- Lattice energy
- Lead
- Leonard Erskine Hill
- Ligand
- Lipid
- Lipophilicity
- Liquid
- Liquid bubble
- Litre
- Log P
- Mass concentration
- Mercury (element)
- Metabolism
- Metallurgy
- Metamorphic rock
- Methanol
- Miscible
- Mixing ratio
- Mixture
- Molality
- Molar concentration
- Molarity
- Mole (unit)
- Mole fraction
- Molecular diffusion
- Naphthalene
- Neutral buoyancy
- Nitrate
- Nitrogen narcosis
- Normocapnia
- Number concentration
- Octanol
- Oligomer
- Organic compounds
- Ostwald ripening
- Oxide
- Oxygen
- Oxygen therapy
- Oxygen toxicity
- Partial molar volume
- Partial pressure
- Paul Bert
- Perfusion
- Permeation
- Petroleum jelly
- PH
- Phase (matter)
- Phase diagram
- Phosphate
- Polar solvent
- Pressure
- Protic solvent
- Raoult's law
- Redox buffer
- Respiratory quotient
- Rubicon Foundation
- Salt
- Salts
- Separatory funnel
- Serial dilution
- Sieverts' law
- Silver
- Silver chloride
- Simon Mitchell
- Simulations Plus
- Sir Robert Boyle
- Sodium chloride
- Sodium sulfate
- Solid
- Solid solution
- Solids
- Solubility
- Solubility chart
- Solubility constant
- Solubility product
- Solubility table
- Solubilization
- Solution
- Solvable group
- Solvation
- Solvation shell
- Solvent
- Solvent extraction
- Solvolysis
- Strontium
- Sulfate
- Sulfide
- Sulfite
- Supersaturation
- Surface tension
- Surfactant
- Synthetic chemistry
- Systemic circulation
- Taravana
- Temperature
- Thallium
- Tissue (biology)
- Torricellian chamber
- Underwater vision
- Uranyl
- Urea
- Valsalva maneuver
- Volume concentration
- Water
- Weight
- William Paul Fife
-

Solubility and intermolecular forces | Chemistry | Khan Academy
Solubility of salt and gas solutes in liquid solvent. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/introduction-to-intermolecular-forces/v/surface-tension?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/introduction-to-intermolecular-forces/v/van-der-waals-forces?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Kh... -

Solubility
070 - Solubility In this video Paul Andersen explains how the dissolution of a solute in a solution can be explained as a reversible reaction. Bonds in the solid solute are broken and the ions are dissolved in a solution. The Ksp (or solubility product constant) can be used to explain the solubility of various salts. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2004, Picture taken by me-- Chris 73 14:12, 11 Dec. English: Solution of Salt in Water (regular Table Salt, Regular Tap water)Esperanto: Salo Solviĝanta En akvoPolski: Sól Ro... -

solubility
-

Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions
CLEAR & SIMPLE Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions - This video explains how to interpret solubility curves and read solubility curves. It discusses saturated, unsaturated and supersaturated solutions. This is an essential component of the unit on solutions/mixtures of substances. In a nutshell, unsaturated solutions have points below the line on the graph, saturated is on the line and supersaturated is above the line on the graph. -

Solubility Rules (Mnemonic Tricks)
Annoyed by all those kids sagging their pants? Well, here's a good way to put that distrubance to use. (It shall help you with your solubility endeavors) -

Solubility product constant from the solubility
How to calculate the solubility (and the molar solubility) of a slightly soluble ionic compound and how to use the solubility to calculate the solubility product constant -

Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7
Dihydrogen monoxide (better know as water) is the key to nearly everything. It falls from the sky, makes up 60% of our bodies, and just about every chemical process related to life takes place with it or in it. Without it, none of the chemical reactions that keep us alive would happen - none of the reactions that sustain any life form on earth would happen - and the majority of inorganic chemical reactions that shape the surface of the earth would not happen either. Every one of us uses water for all kinds of chemistry every day - our body chemistry, our food chemistry and our laundry chemistry all take place in water. In today's Crash Course Chemistry, we use Hank's actual dirty laundry (ew) to learn about some of the properties of water that make it so special - it's polarity and diele... -

Factors that Affect Solubility - CLEAR & SIMPLE
CLEAR & SIMPLE - Solubility of Substances - What Changes the Solubility of a Substance - Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions. This video explains the factors that affect solubility. The video explains the relationship between heat-temperature and solubility, pressure and solubility, surface area and solubility, agitation-shaking and solubility and the type of solvent, ionic, polar covalent, nonpolar covalent. -

Solubility Explained
In this video I will explain the how and why different substances dissolve in water. I will also explain the polar nature of water -

Solubility of Ionic Compounds: Basics and Rules
Basic discussion on the solubility of ionic compounds and the rules for determining whether an ionic compound is soluble or insoluble. -

Solubility Rules and Precipitation Reactions - Mr. Causey's Chemistry
Mr. Causey discusses the solubility rules and the importance of knowing these rules in order to predict if a precipitate will form during a reaction. http://www.yourCHEMcoach.com Learn more and understand better with Mr. Causey's tutorials. Subscribe for more chemistry videos: http://bit.ly/1jeutVl Share this Video: https://www.youtube.com/watch?v=UbyqA5mi6GU Resources: Polyatomic Ion Cheat Sheet: http://bit.ly/14e2pbw Related Videos: Aqueous Solutions: http://www.youtube.com/watch?v=vg5FUNIrW3o Net Ionic Equations: http://www.youtube.com/watch?v=vbx5nfAiBlM Contact Me: mrcausey@mrcausey.com Follow Me: http://www.twitter.com/#!/mrcausey http://pinterest.com/mistercausey/ http://www.facebook.com/profile.php?id=814523544 In order to predict if a precipitate will form during a reactio... -

Solubility Song
Solubility Rules in the form of a song -

The Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr Pauller
This video shows a demonstration which illustrates how the solubility of carbon dioxide in water changes due to changes in water temperature. -

Solutions Lesson 1 Solutions and Solubility
-

What is Ksp? (Solubility Product Constant)
Ksp is really just an equilibrium constant (Keq), but it's for a solid dissolving in water. This is special, since all of the reactants are solid, and so they AREN'T included in the equilibrium expression. -

Soluble and insoluble materials - Experiment - Elementary Science
This is an elementary science video for kids that help them understand and distinguish soluble and insoluble materials. SOLUBLE AND INSOLUBLE MATERIALS: To check the solubility of substances take three glasses of water a spoon and some salt, sugar and sand. Add a spoon of salt to the first glass and stir it the salt completely. Notice that the salt completely dissolves in the water. Similarly, add a spoon of sugar to the second glass. Observe that sugar also dissolves in the water. Finally add the sand sample to the glass of water the sand will not dissolve in the water and just settles at the bottom of the glass. Materials like salt and sugar will dissolve in the water and are called soluble as they dissolve completely in the water, where as substances that do not dissolve in water like ... -

Solubility Rules (Call Me Maybe Parody)
Professor Lichter , Chm 1045 Fall 2012 MWF 11-12:15 Lyrics: All compounds of group 1 elements Are soluble Man they're heaven sent All ammonium salts that's NH4+ Are soluble Makes me want some more All nitrates, chlorates, perchlorates, and even acetates Salts are soluble that's crazy! Hey Chemistry is fun Practice daily Read your textbook And get an A maybe This is solubility It's so crazy Don't be a fool And follow these rules Hey Chemistry is fun Practice daily Read your textbook And get an A maybe This is solubility It's so crazy Don't be a fool And follow these rules All chloride, bromide, ---and iodide Salts are soluble Its like I'm on a fun slide Some exceptions OMG Silver, lead, Mercury They don't want to be free Their insoluble All sulfates soluble Barium, strontium,... -

Solubility Curves Explained
IN this video I will explain solubility curves and provide a couple of examples using solubility curves. -

Solubility of organic compounds
How to determine whether or not an organic compound dissolves in water -

Factors Affecting Solubility
Follow us at: https://plus.google.com/+tutorvista/ Check us out at http://chemistry.tutorvista.com/inorganic-chemistry/solubility-rules.html FACTORS AFFECTING SOLUBILITY There are three main factors that control solubility of a solute. (1) Temperature (2) Nature of solute or solvent (3) Pressure EFFECT OF TEMPERATURE Generally in many cases solubility increases with the rise in temperature and decreases with the fall of temperature but it is not necessary in all cases. However we must follow two behaviors: In endothermic process solubility increases with the increase in temperature and vice versa. For example: solubility of potassium nitrate increases with the increase in . temperature. In exothermic process solubility decrease with the inc... -

Solubility Curves | Chemistry for All | The Fuse School
Learn the basics about solubility curves as a part of the overall properties of matter topic. SUBSCRIBE to the Fuse School YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT. JOIN our platform at www.fuseschool.org This video is part of 'Chemistry for All' - a Chemistry Education project by our Charity Fuse Foundation - the organisation behind The Fuse School. These videos can be used in a flipped classroom model or as a revision aid. Find our other Chemistry videos here: https://www.youtube.com/playlist?list=PLW0gavSzhMlReKGMVfUt6YuNQsO0bqSMV Twitter: https://twitter.com/fuseSchool Access a deeper Learning Experience in the Fuse School platform and app: www.fus... -

Solubility and the common-ion effect
How the common-ion effect decreases the solubility of a slightly soluble compound -

Compare solubility of salt, sugar and chalk | Solutions | Chemistry
This activity shows that it is possible to use the nature of the physical structure of a substance to predict if it will dissolve in water or not. While this is not guaranteed every time, it gives a fair indication. Crystalline or grainy substances like salt and sugar tend to be water soluble, whereas coarse dry powders like chalk are insoluble.
Solubility and intermolecular forces | Chemistry | Khan Academy
- Order: Reorder
- Duration: 13:22
- Updated: 03 Sep 2009
- views: 378750
- published: 03 Sep 2009
- views: 378750
Solubility
- Order: Reorder
- Duration: 7:06
- Updated: 26 Feb 2014
- views: 52032
- published: 26 Feb 2014
- views: 52032
solubility
- Order: Reorder
- Duration: 3:32
- Updated: 20 Jan 2012
- views: 5289
- published: 20 Jan 2012
- views: 5289
Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions
- Order: Reorder
- Duration: 14:15
- Updated: 10 May 2012
- views: 61707
- published: 10 May 2012
- views: 61707
Solubility Rules (Mnemonic Tricks)
- Order: Reorder
- Duration: 2:41
- Updated: 27 Jul 2012
- views: 198790
- published: 27 Jul 2012
- views: 198790
Solubility product constant from the solubility
- Order: Reorder
- Duration: 11:45
- Updated: 21 Sep 2014
- views: 34422
- published: 21 Sep 2014
- views: 34422
Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7
- Order: Reorder
- Duration: 13:34
- Updated: 26 Mar 2013
- views: 598325
- published: 26 Mar 2013
- views: 598325
Factors that Affect Solubility - CLEAR & SIMPLE
- Order: Reorder
- Duration: 9:09
- Updated: 08 May 2012
- views: 12763
- published: 08 May 2012
- views: 12763
Solubility Explained
- Order: Reorder
- Duration: 13:55
- Updated: 02 Apr 2015
- views: 2424
- published: 02 Apr 2015
- views: 2424
Solubility of Ionic Compounds: Basics and Rules
- Order: Reorder
- Duration: 5:14
- Updated: 10 Jun 2011
- views: 68964
- published: 10 Jun 2011
- views: 68964
Solubility Rules and Precipitation Reactions - Mr. Causey's Chemistry
- Order: Reorder
- Duration: 6:37
- Updated: 18 Mar 2014
- views: 35752
- published: 18 Mar 2014
- views: 35752
Solubility Song
- Order: Reorder
- Duration: 1:22
- Updated: 03 Nov 2009
- views: 126648
The Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr Pauller
- Order: Reorder
- Duration: 3:15
- Updated: 17 Apr 2015
- views: 2788
- published: 17 Apr 2015
- views: 2788
Solutions Lesson 1 Solutions and Solubility
- Order: Reorder
- Duration: 21:02
- Updated: 10 Mar 2012
- views: 27739
- published: 10 Mar 2012
- views: 27739
What is Ksp? (Solubility Product Constant)
- Order: Reorder
- Duration: 3:40
- Updated: 28 Mar 2012
- views: 115803
- published: 28 Mar 2012
- views: 115803
Soluble and insoluble materials - Experiment - Elementary Science
- Order: Reorder
- Duration: 1:27
- Updated: 05 Sep 2012
- views: 48771
- published: 05 Sep 2012
- views: 48771
Solubility Rules (Call Me Maybe Parody)
- Order: Reorder
- Duration: 3:17
- Updated: 10 Oct 2012
- views: 69926
- published: 10 Oct 2012
- views: 69926
Solubility Curves Explained
- Order: Reorder
- Duration: 15:10
- Updated: 03 Apr 2015
- views: 2670
- published: 03 Apr 2015
- views: 2670
Solubility of organic compounds
- Order: Reorder
- Duration: 12:21
- Updated: 17 May 2015
- views: 21344
- published: 17 May 2015
- views: 21344
Factors Affecting Solubility
- Order: Reorder
- Duration: 3:39
- Updated: 05 May 2010
- views: 33312
- published: 05 May 2010
- views: 33312
Solubility Curves | Chemistry for All | The Fuse School
- Order: Reorder
- Duration: 4:24
- Updated: 07 Jan 2016
- views: 1808
- published: 07 Jan 2016
- views: 1808
Solubility and the common-ion effect
- Order: Reorder
- Duration: 8:15
- Updated: 27 Sep 2014
- views: 23060
- published: 27 Sep 2014
- views: 23060
Compare solubility of salt, sugar and chalk | Solutions | Chemistry
- Order: Reorder
- Duration: 2:06
- Updated: 19 Jun 2013
- views: 15263
- published: 19 Jun 2013
- views: 15263
- Playlist
- Chat
- Playlist
- Chat

Solubility and intermolecular forces | Chemistry | Khan Academy
- Report rights infringement
- published: 03 Sep 2009
- views: 378750

Solubility
- Report rights infringement
- published: 26 Feb 2014
- views: 52032

solubility
- Report rights infringement
- published: 20 Jan 2012
- views: 5289

Solubility Curves - Saturated, Unsaturated, Supersaturated Solutions
- Report rights infringement
- published: 10 May 2012
- views: 61707

Solubility Rules (Mnemonic Tricks)
- Report rights infringement
- published: 27 Jul 2012
- views: 198790

Solubility product constant from the solubility
- Report rights infringement
- published: 21 Sep 2014
- views: 34422

Water and Solutions -- for Dirty Laundry: Crash Course Chemistry #7
- Report rights infringement
- published: 26 Mar 2013
- views: 598325

Factors that Affect Solubility - CLEAR & SIMPLE
- Report rights infringement
- published: 08 May 2012
- views: 12763

Solubility Explained
- Report rights infringement
- published: 02 Apr 2015
- views: 2424

Solubility of Ionic Compounds: Basics and Rules
- Report rights infringement
- published: 10 Jun 2011
- views: 68964

Solubility Rules and Precipitation Reactions - Mr. Causey's Chemistry
- Report rights infringement
- published: 18 Mar 2014
- views: 35752

Solubility Song
- Report rights infringement
- published: 03 Nov 2009
- views: 126648

The Effect of Temperature on the Solubility of Gases - A Science Experiment with Mr Pauller
- Report rights infringement
- published: 17 Apr 2015
- views: 2788

Solutions Lesson 1 Solutions and Solubility
- Report rights infringement
- published: 10 Mar 2012
- views: 27739
-
Lyrics list:text lyricsplay full screenplay karaoke
Journalist Says Saudis Paid Pakistan To Hide Bin Laden From Americans
Edit WorldNews.com 21 Apr 2016Wrestling star Chyna found dead at Redondo Beach home
Edit Orlando Sentinel 21 Apr 2016Jennifer Aniston DECLARED World’s Most Beautiful Woman and we couldn’t agree more!
Edit Bollywood Life 21 Apr 2016Increased Activity At North Korean Nuclear Site May Indicate Impending Test
Edit WorldNews.com 21 Apr 2016No More Boring Oatmeal!
Edit Palm Beach Post 21 Apr 2016Camel milk for soft skin
Edit DNA India 21 Apr 2016USGC Assesses Demand for DDGS, Sorghum in Turkey (US Grains Council)
Edit Public Technologies 21 Apr 2016March 2016 Quarterly Activities Report (Tiger Resources Limited)
Edit Public Technologies 21 Apr 2016One patent, many applications (The University of Mississippi Medical Center)
Edit Public Technologies 21 Apr 2016Beans: The Super Food that Keeps You Full
Edit Palm Beach Post 21 Apr 2016When a global Swiss agribusiness giant becomes Chinese-owned
Edit Indian Express 21 Apr 2016Technology and business: When a global Swiss agribusiness giant becomes Chinese-owned
Edit Yahoo Daily News 21 Apr 201612 Fruits And Veggies With The Most Pesticides
Edit OlWomen 21 Apr 2016Water Soluble Fertilizers Market to Grow at 5% CAGR TechSci Research Report
Edit PR Newswire 20 Apr 201650% Of The Great Barrier Reef Is Now Dead Or Dying, 93% Is Bleached
Edit IFL Science 20 Apr 2016Severe Arctic Ocean acidification via permafrost thawing and river runoff (Stockholms Universitet)
Edit Public Technologies 20 Apr 2016- 1
- 2
- 3
- 4
- 5
- Next page »







