- published: 16 Sep 2014
- views: 913309
-
remove the playlistUncertainty_principle
- remove the playlistUncertainty_principle
- published: 14 Jan 2013
- views: 1102482
- published: 18 Dec 2014
- views: 200905
- published: 09 Sep 2016
- views: 15518
- published: 25 Jun 2014
- views: 108644
- published: 31 Jul 2011
- views: 1983065
- published: 23 Jan 2010
- views: 364897
- published: 08 Apr 2013
- views: 38511
- published: 14 Jun 2012
- views: 480172

Uncertainty principle
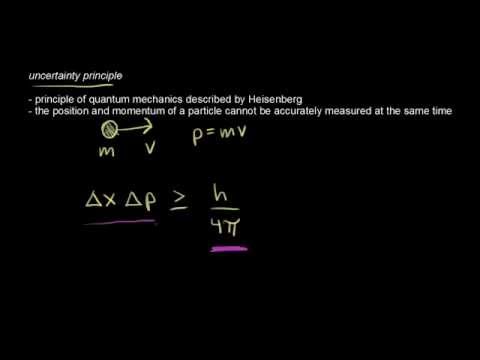
In quantum mechanics, the uncertainty principle, also known as Heisenberg's uncertainty principle, is any of a variety of mathematical inequalities asserting a fundamental limit to the precision with which certain pairs of physical properties of a particle, known as complementary variables, such as position x and momentum p, can be known simultaneously.
Introduced first in 1927, by the German physicist Werner Heisenberg, it states that the more precisely the position of some particle is determined, the less precisely its momentum can be known, and vice versa. The formal inequality relating the standard deviation of position σx and the standard deviation of momentum σp was derived by Earle Hesse Kennard later that year and by Hermann Weyl in 1928:
(ħ is the reduced Planck constant, h / 2π).
Historically, the uncertainty principle has been confused with a somewhat similar effect in physics, called the observer effect, which notes that measurements of certain systems cannot be made without affecting the systems. Heisenberg offered such an observer effect at the quantum level (see below) as a physical "explanation" of quantum uncertainty. It has since become clear, however, that the uncertainty principle is inherent in the properties of all wave-like systems, and that it arises in quantum mechanics simply due to the matter wave nature of all quantum objects. Thus, the uncertainty principle actually states a fundamental property of quantum systems, and is not a statement about the observational success of current technology. It must be emphasized that measurement does not mean only a process in which a physicist-observer takes part, but rather any interaction between classical and quantum objects regardless of any observer.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 4:44
4:44What is the Heisenberg Uncertainty Principle? - Chad Orzel
What is the Heisenberg Uncertainty Principle? - Chad OrzelWhat is the Heisenberg Uncertainty Principle? - Chad Orzel
View full lesson: http://ed.ted.com/lessons/what-is-the-heisenberg-uncertainty-principle-chad-orzel The Heisenberg Uncertainty Principle states that you can never simultaneously know the exact position and the exact speed of an object. Why not? Because everything in the universe behaves like both a particle and a wave at the same time. Chad Orzel navigates this complex concept of quantum physics. Lesson by Chad Orzel, animation by Henrik Malmgren. -
 4:12
4:12Heisenberg's Uncertainty Principle Explained
Heisenberg's Uncertainty Principle ExplainedHeisenberg's Uncertainty Principle Explained
Heisenberg's uncertainty principle tells us that it is impossible to simultaneously measure the position and momentum of a particle with infinite precision. In our everyday lives we virtually never come up against this limit, hence why it seems peculiar. In this experiment a laser is shone through a narrow slit onto a screen. As the slit is made narrower, the spot on the screen also becomes narrower. But at a certain point, the spot starts becoming wider. This is because the photons of light have been so localised at the slit that their horizontal momentum must become less well defined in order to satisfy Heisenberg's uncertainty principle. I based this video on one by Prof. Walter Lewin of MIT: http://bit.ly/100Wk2K Henry (MinutePhysics) has previously made a video about Heisenberg's Uncertainty Principle where he treats it as less spooky and more a consequence of waves: http://bit.ly/TV3xO5 Sixty Symbols has a great video on Planck's constant: http://bit.ly/11upebY Thanks to the University of Sydney for hosting this experiment, especially to Tom and Ralph for their assistance getting it working. Music: Kevin McLeod (Incompetech.com) Mirage and Danse Macabre -
 48:01
48:01Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)
Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)
Classical Mechanics, in spite of all of its impressive predictive power, fails to explain many microscopic behaviors. This led to the development of Quantum Mechanics, where electrons orbit nuclei in discrete energy levels, light can behave as a particle, and particles behave as waves. The location of microscopic particles can only be expressed in terms of probabilities. Heisenberg's uncertainty principle is discussed and demonstrated. This lecture is part of 8.01 Physics I: Classical Mechanics, as taught in Fall 1999 by Dr. Walter Lewin at MIT. This video was formerly hosted on the YouTube channel MIT OpenCourseWare. This version was downloaded from the Internet Archive, at https://archive.org/details/MIT8.01F99/. Attribution: MIT OpenCourseWare License: Creative Commons BY-NC-SA 3.0 US To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/us/. More information at http://ocw.mit.edu/terms/. This YouTube channel is independently operated. It is neither affiliated with nor endorsed by MIT, MIT OpenCourseWare, the Internet Archive, or Dr. Lewin. -
 8:56
8:56What Heisenberg's Uncertainty Principle *Actually* Means
What Heisenberg's Uncertainty Principle *Actually* MeansWhat Heisenberg's Uncertainty Principle *Actually* Means
Let's talk about one of the most misunderstood but awesome concepts in physics. -
 10:18
10:18Heisenberg uncertainty principle | Chemistry | Khan Academy
Heisenberg uncertainty principle | Chemistry | Khan AcademyHeisenberg uncertainty principle | Chemistry | Khan Academy
Definition of Heisenberg uncertainty principle. Calculating uncertainty in position given the uncertainty in momentum for Bohr model of hydrogen. Created by Jay. Watch the next lesson: https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/orbitals-and-electrons/v/quantum-numbers?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/bohr-model-hydrogen/v/absorption-and-emission?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Chemistry channel: https://www.youtube.com/channel/UCyEot66LrwWFEMONvrIBh3A?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
 1:04
1:04What is the Uncertainty Principle?
What is the Uncertainty Principle?What is the Uncertainty Principle?
The Heisenberg uncertainty principle - in a nutshell! Tweet it - http://bit.ly/pagxNi Facebook it - http://on.fb.me/pzzfqM minutephysics is now on Google+ - http://bit.ly/qzEwc6 And facebook - http://facebook.com/minutephysics Minute Physics provides an energetic and entertaining view of old and new problems in physics -- all in a minute! In this episode, we talk about the Heisenberg uncertainty principle and how it's not really that weird - it's just a property of waves! Music by Nathaniel Schroeder youtube: http://bit.ly/pakJLE myspace: http://mysp.ac/qtmZQj Created by Henry Reich -
 5:49
5:49Quantum Mechanics: The Uncertainty Principle
Quantum Mechanics: The Uncertainty PrincipleQuantum Mechanics: The Uncertainty Principle
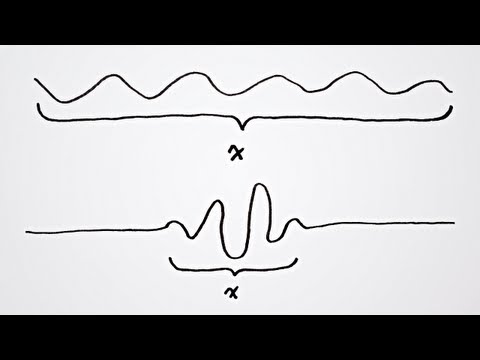
http://www.facebook.com/ScienceReason ... Quantum Mechanics (Chapter 4): The Heisenberg Uncertainty Principle. --- Please SUBSCRIBE to Science & Reason: • http://www.youtube.com/Best0fScience • http://www.youtube.com/ScienceTV • http://www.youtube.com/FFreeThinker --- 1. A Brief History Of Quantum Mechanics http://www.youtube.com/watch?v=B7pACq_xWyw 2. The Structure Of Atoms http://www.youtube.com/watch?v=-YYBCNQnYNM 3. Wave Function And Wave-Particle Duality http://www.youtube.com/watch?v=7GTCus7KTb0 4. The Uncertainty Principle http://www.youtube.com/watch?v=Fw6dI7cguCg 5. The Spin Of Fundamental Particles 6. Quantum Entanglement --- In quantum mechanics, the Heisenberg uncertainty principle states that certain pairs of physical properties, like position and momentum, cannot both be known to arbitrary precision. That is, the more precisely one property is known, the less precisely the other can be known. This statement has been interpreted in two different ways. According to Heisenberg its meaning is that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty. According to others (for instance Ballentine) this is not a statement about the limitations of a researcher's ability to measure particular quantities of a system, but it is a statement about the nature of the system itself as described by the equations of quantum mechanics. In quantum physics, a particle is described by a wave packet, which gives rise to this phenomenon. Consider the measurement of the absolute position of a particle. It could be anywhere the particle's wave packet has non-zero amplitude, meaning the position is uncertain - it could be almost anywhere along the wave packet. To obtain an accurate reading of position, this wave packet must be 'compressed' as much as possible, meaning it must be made up of increasing numbers of sine waves added together. The momentum of the particle is proportional to the wavelength of one of these waves, but it could be any of them. So a more accurate position measurementby adding together more wavesmeans the momentum measurement becomes less accurate (and vice versa). The only kind of wave with a definite position is concentrated at one point, and such a wave has an indefinite wavelength (and therefore an indefinite momentum). Conversely, the only kind of wave with a definite wavelength is an infinite regular periodic oscillation over all space, which has no definite position. So in quantum mechanics, there can be no states that describe a particle with both a definite position and a definite momentum. The more precise the position, the less precise the momentum. The uncertainty principle can be restated in terms of measurements, which involves collapse of the wavefunction. When the position is measured, the wavefunction collapses to a narrow bump near the measured value, and the momentum wavefunction becomes spread out. The particle's momentum is left uncertain by an amount inversely proportional to the accuracy of the position measurement. The amount of left-over uncertainty can never be reduced below the limit set by the uncertainty principle, no matter what the measurement process. This means that the uncertainty principle is related to the observer effect, with which it is often conflated. The uncertainty principle sets a lower limit to how small the momentum disturbance in an accurate position experiment can be, and vice versa for momentum experiments. • http://en.wikipedia.org/wiki/Uncertainty_principle --- The Cassiopeia Project - making science simple! The Cassiopeia Project is an effort to make high quality science videos available to everyone. If you can visualize it, then understanding is not far behind. • http://www.cassiopeiaproject.com . -
 16:15
16:15Heisenberg Uncertainty Principle Derived and Explained | Doc Physics
Heisenberg Uncertainty Principle Derived and Explained | Doc PhysicsHeisenberg Uncertainty Principle Derived and Explained | Doc Physics
One of the most-oft quoted results of quantum physics, this doozie forces us to reconsider what we can know about the universe. Some things cannot be known simultaneously. In fact, if anything about a system is known perfectly, there is likely another characteristic that is completely shrouded in uncertainty. So significant figures ARE important after all! I should point out that, although my derivation is easy to understand conceptually, it is incomplete and its result is not perfect. A proper (but messier) treatment yields a minimum uncertainty of h/(4*pi). -
 64:07
64:07Quantization and Heisenberg's Uncertainty Principle
Quantization and Heisenberg's Uncertainty PrincipleQuantization and Heisenberg's Uncertainty Principle
Quantization and Heisenberg's Uncertainty Principle -
 3:16
3:16IDTIMWYTIM: Heisenberg Uncertainty Principle
IDTIMWYTIM: Heisenberg Uncertainty PrincipleIDTIMWYTIM: Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle might not mean what you think it means: Hank clears things up for us in this edition of IDTIMWYTIM, by distinguishing between the Uncertainty Principle and the Observer Effect, which are often conflated. Like SciShow on Facebook: http://www.facebook.com/scishow Follow SciShow on Twitter: http://www.twitter.com/scishow References: http://hyperphysics.phy-astr.gsu.edu/hbase/uncer.html http://science.howstuffworks.com/innovation/science-questions/quantum-suicide2.htm http://www.aip.org/history/heisenberg/p08.htm
-

What is the Heisenberg Uncertainty Principle? - Chad Orzel
View full lesson: http://ed.ted.com/lessons/what-is-the-heisenberg-uncertainty-principle-chad-orzel The Heisenberg Uncertainty Principle states that you can never simultaneously know the exact position and the exact speed of an object. Why not? Because everything in the universe behaves like both a particle and a wave at the same time. Chad Orzel navigates this complex concept of quantum physics. Lesson by Chad Orzel, animation by Henrik Malmgren.
published: 16 Sep 2014 -

Heisenberg's Uncertainty Principle Explained
Heisenberg's uncertainty principle tells us that it is impossible to simultaneously measure the position and momentum of a particle with infinite precision. In our everyday lives we virtually never come up against this limit, hence why it seems peculiar. In this experiment a laser is shone through a narrow slit onto a screen. As the slit is made narrower, the spot on the screen also becomes narrower. But at a certain point, the spot starts becoming wider. This is because the photons of light have been so localised at the slit that their horizontal momentum must become less well defined in order to satisfy Heisenberg's uncertainty principle. I based this video on one by Prof. Walter Lewin of MIT: http://bit.ly/100Wk2K Henry (MinutePhysics) has previously made a video about Heisenberg's Un...
published: 14 Jan 2013 -

Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)
Classical Mechanics, in spite of all of its impressive predictive power, fails to explain many microscopic behaviors. This led to the development of Quantum Mechanics, where electrons orbit nuclei in discrete energy levels, light can behave as a particle, and particles behave as waves. The location of microscopic particles can only be expressed in terms of probabilities. Heisenberg's uncertainty principle is discussed and demonstrated. This lecture is part of 8.01 Physics I: Classical Mechanics, as taught in Fall 1999 by Dr. Walter Lewin at MIT. This video was formerly hosted on the YouTube channel MIT OpenCourseWare. This version was downloaded from the Internet Archive, at https://archive.org/details/MIT8.01F99/. Attribution: MIT OpenCourseWare License: Creative Commons BY-NC-SA 3.0 U...
published: 18 Dec 2014 -

What Heisenberg's Uncertainty Principle *Actually* Means
Let's talk about one of the most misunderstood but awesome concepts in physics.
published: 09 Sep 2016 -

Heisenberg uncertainty principle | Chemistry | Khan Academy
Definition of Heisenberg uncertainty principle. Calculating uncertainty in position given the uncertainty in momentum for Bohr model of hydrogen. Created by Jay. Watch the next lesson: https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/orbitals-and-electrons/v/quantum-numbers?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/bohr-model-hydrogen/v/absorption-and-emission?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a...
published: 25 Jun 2014 -

What is the Uncertainty Principle?
The Heisenberg uncertainty principle - in a nutshell! Tweet it - http://bit.ly/pagxNi Facebook it - http://on.fb.me/pzzfqM minutephysics is now on Google+ - http://bit.ly/qzEwc6 And facebook - http://facebook.com/minutephysics Minute Physics provides an energetic and entertaining view of old and new problems in physics -- all in a minute! In this episode, we talk about the Heisenberg uncertainty principle and how it's not really that weird - it's just a property of waves! Music by Nathaniel Schroeder youtube: http://bit.ly/pakJLE myspace: http://mysp.ac/qtmZQj Created by Henry Reich
published: 31 Jul 2011 -

Quantum Mechanics: The Uncertainty Principle
http://www.facebook.com/ScienceReason ... Quantum Mechanics (Chapter 4): The Heisenberg Uncertainty Principle. --- Please SUBSCRIBE to Science & Reason: • http://www.youtube.com/Best0fScience • http://www.youtube.com/ScienceTV • http://www.youtube.com/FFreeThinker --- 1. A Brief History Of Quantum Mechanics http://www.youtube.com/watch?v=B7pACq_xWyw 2. The Structure Of Atoms http://www.youtube.com/watch?v=-YYBCNQnYNM 3. Wave Function And Wave-Particle Duality http://www.youtube.com/watch?v=7GTCus7KTb0 4. The Uncertainty Principle http://www.youtube.com/watch?v=Fw6dI7cguCg 5. The Spin Of Fundamental Particles 6. Quantum Entanglement --- In quantum mechanics, the Heisenberg uncertainty principle states that certain pairs of physical properties, like position and momentum, cannot both be ...
published: 23 Jan 2010 -

Heisenberg Uncertainty Principle Derived and Explained | Doc Physics
One of the most-oft quoted results of quantum physics, this doozie forces us to reconsider what we can know about the universe. Some things cannot be known simultaneously. In fact, if anything about a system is known perfectly, there is likely another characteristic that is completely shrouded in uncertainty. So significant figures ARE important after all! I should point out that, although my derivation is easy to understand conceptually, it is incomplete and its result is not perfect. A proper (but messier) treatment yields a minimum uncertainty of h/(4*pi).
published: 08 Apr 2013 -

Quantization and Heisenberg's Uncertainty Principle
Quantization and Heisenberg's Uncertainty Principle
published: 17 May 2015 -

IDTIMWYTIM: Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle might not mean what you think it means: Hank clears things up for us in this edition of IDTIMWYTIM, by distinguishing between the Uncertainty Principle and the Observer Effect, which are often conflated. Like SciShow on Facebook: http://www.facebook.com/scishow Follow SciShow on Twitter: http://www.twitter.com/scishow References: http://hyperphysics.phy-astr.gsu.edu/hbase/uncer.html http://science.howstuffworks.com/innovation/science-questions/quantum-suicide2.htm http://www.aip.org/history/heisenberg/p08.htm
published: 14 Jun 2012
What is the Heisenberg Uncertainty Principle? - Chad Orzel
- Order: Reorder
- Duration: 4:44
- Updated: 16 Sep 2014
- views: 913309
- published: 16 Sep 2014
- views: 913309
Heisenberg's Uncertainty Principle Explained
- Order: Reorder
- Duration: 4:12
- Updated: 14 Jan 2013
- views: 1102482
- published: 14 Jan 2013
- views: 1102482
Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)
- Order: Reorder
- Duration: 48:01
- Updated: 18 Dec 2014
- views: 200905
- published: 18 Dec 2014
- views: 200905
What Heisenberg's Uncertainty Principle *Actually* Means
- Order: Reorder
- Duration: 8:56
- Updated: 09 Sep 2016
- views: 15518
- published: 09 Sep 2016
- views: 15518
Heisenberg uncertainty principle | Chemistry | Khan Academy
- Order: Reorder
- Duration: 10:18
- Updated: 25 Jun 2014
- views: 108644
- published: 25 Jun 2014
- views: 108644
What is the Uncertainty Principle?
- Order: Reorder
- Duration: 1:04
- Updated: 31 Jul 2011
- views: 1983065
- published: 31 Jul 2011
- views: 1983065
Quantum Mechanics: The Uncertainty Principle
- Order: Reorder
- Duration: 5:49
- Updated: 23 Jan 2010
- views: 364897
- published: 23 Jan 2010
- views: 364897
Heisenberg Uncertainty Principle Derived and Explained | Doc Physics
- Order: Reorder
- Duration: 16:15
- Updated: 08 Apr 2013
- views: 38511
- published: 08 Apr 2013
- views: 38511
Quantization and Heisenberg's Uncertainty Principle
- Order: Reorder
- Duration: 64:07
- Updated: 17 May 2015
- views: 14566
IDTIMWYTIM: Heisenberg Uncertainty Principle
- Order: Reorder
- Duration: 3:16
- Updated: 14 Jun 2012
- views: 480172
- published: 14 Jun 2012
- views: 480172
- Playlist
- Chat
- Playlist
- Chat

What is the Heisenberg Uncertainty Principle? - Chad Orzel
- Report rights infringement
- published: 16 Sep 2014
- views: 913309

Heisenberg's Uncertainty Principle Explained
- Report rights infringement
- published: 14 Jan 2013
- views: 1102482

Lec 34: Heisenberg's Uncertainty Principle | 8.01 Classical Mechanics, Fall 1999 (Walter Lewin)
- Report rights infringement
- published: 18 Dec 2014
- views: 200905

What Heisenberg's Uncertainty Principle *Actually* Means
- Report rights infringement
- published: 09 Sep 2016
- views: 15518

Heisenberg uncertainty principle | Chemistry | Khan Academy
- Report rights infringement
- published: 25 Jun 2014
- views: 108644

What is the Uncertainty Principle?
- Report rights infringement
- published: 31 Jul 2011
- views: 1983065

Quantum Mechanics: The Uncertainty Principle
- Report rights infringement
- published: 23 Jan 2010
- views: 364897

Heisenberg Uncertainty Principle Derived and Explained | Doc Physics
- Report rights infringement
- published: 08 Apr 2013
- views: 38511

Quantization and Heisenberg's Uncertainty Principle
- Report rights infringement
- published: 17 May 2015
- views: 14566

IDTIMWYTIM: Heisenberg Uncertainty Principle
- Report rights infringement
- published: 14 Jun 2012
- views: 480172
[VIDEO]: Hillary Clinton Concession Speech: 'I'm Sorry We Did Not Win'
Edit WorldNews.com 09 Nov 2016Mexican Foreign Minister: We Will Not Pay For Border Wall
Edit WorldNews.com 09 Nov 2016Here's the full text of Donald Trump's victory speech
Edit CNN 09 Nov 2016The Middle East will present Donald Trump with a terrifying choice – and he won't be able to handle it
Edit The Independent 09 Nov 2016A word from Bernie voters: #DontBlameMeIVotedForBernie
Edit Al Jazeera 09 Nov 2016Xi congratulates victor Trump
Edit China Daily 10 Nov 2016World resigned, but wary of unpredictable Donald Trump
Edit The Oklahoman 10 Nov 2016Foreign leaders react to President-elect Trump
Edit The Oklahoman 10 Nov 2016World leaders welcome, but seek clarity on Trump policies
Edit Arabnews 10 Nov 2016S&P; affirms U.S. investment-grade ratings after presidential election
Edit Yahoo Daily News 10 Nov 2016The Election (Colorado State University)
Edit Public Technologies 10 Nov 2016Summary of Consolidated Financial Results [Japanese GAAP] For the Second Quarter of the Fiscal Year Ending March 31, 2017 [158KB] (Nippon Kayaku Co Ltd)
Edit Public Technologies 10 Nov 2016New Zealand central bank cuts interest rate to record low 1.75 percent
Edit Xinhua 10 Nov 2016Taiwan's Ruling Party Urges China to Respect Hong Kong's Democratic Aspirations
Edit Topix 10 Nov 2016Property shortage 'fuelling' Scottish house price rises
Edit BBC News 10 Nov 2016Honduran President to Seek a Second Term; Opposition Cries Foul
Edit Voa News 10 Nov 2016- 1
- 2
- 3
- 4
- 5
- Next page »








