- published: 23 Feb 2015
- views: 17764
-
remove the playlistEmission Line
-
remove the playlistLongest Videos
- remove the playlistEmission Line
- remove the playlistLongest Videos
- published: 29 Apr 2013
- views: 8310
- published: 06 Mar 2015
- views: 58643
- published: 26 May 2012
- views: 47775
- published: 16 Nov 2014
- views: 2343
- published: 23 Jun 2014
- views: 79617
- published: 02 May 2015
- views: 353
- published: 05 Oct 2014
- views: 7782
- published: 27 Feb 2016
- views: 1905
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state.
Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify the elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
In physics, emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emission of a photon, resulting in the production of light. The frequency of light emitted is a function of the energy of the transition. Since energy must be conserved, the energy difference between the two states equals the energy carried off by the photon. The energy states of the transitions can lead to emissions over a very large range of frequencies. For example, visible light is emitted by the coupling of electronic states in atoms and molecules (then the phenomenon is called fluorescence or phosphorescence). On the other hand, nuclear shell transitions can emit high energy gamma rays, while nuclear spin transitions emit low energy radio waves.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 5:12
5:12A Level Physics - Emission and Absorption Line Spectra
A Level Physics - Emission and Absorption Line SpectraA Level Physics - Emission and Absorption Line Spectra
Why are fireworks coloured, how can we tell what elements are in stars just from the light we see? This video explains why physics is all about sunshine and rainbows. If you would like to see more A Level Physics videos then please Subscribe to my channel to keep updated with new videos and to search the Playlists already created. You can also visit my site 'A Level Physics Online' to see how all the videos relate to your course and for even more resources at http://www.alevelphysicsonline.com/ Thanks for watching, Mr Matheson -
 3:33
3:33Emission and absorption spectra
Emission and absorption spectraEmission and absorption spectra
A video explaining what emission and absorption spectra are and how they are created. This does not go into the mechanics of the Bohr atom and electron excitation. -
 5:18
5:18Emission and Absorption Spectra
Emission and Absorption SpectraEmission and Absorption Spectra
086 - Emission and Absorption Spectra In this video Paul Andersen explains how the photons emitted from or absorbed by an atom or nuclei is directly related to electrons moving between energy level. Absorption and emission are a direct result of the conservation of energy. Scientists may use the emission spectra of material to determine the atoms or molecules contained within the material. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: English: Colorful Spectrum simulationEspañol: Espectro de Colores de La Luz visibleEsperanto: Koloroj Videblaj中文(简体): 软件模拟的可见光谱。中文(繁體): 軟件模擬的可見光譜。, August 27, 2008. Digitally created by Deborah S Krolls, December 13, 2004. http://commons.wikimedia.org/wiki/File:Spectrum4websiteEval.png. “Gas-Filled Tube.” Wikipedia, the Free Encyclopedia, January 31, 2015. http://en.wikipedia.org/w/index.php?title=Gas-filled_tube&oldid;=634245710. Kuiper, Pieter. English: Fridge Letters in Natural Light, November 9, 2009. Own work. http://commons.wikimedia.org/wiki/File:Fluorescence_off.jpg. ———. English: Plastic Fridge Letters (from Different Manufacturers) When a Fluorescent Lamp (Labino) Is Switched on, November 9, 2009. Own work. http://commons.wikimedia.org/wiki/File:Fluorescence_on.jpg. Kurzon. English: A Simple Illustration of Bohr’s Model of the Atom, with an Electron Making Quantum Leaps., April 14, 2014. Own work. http://commons.wikimedia.org/wiki/File:Bohr_atom_animation.gif. -
 12:21
12:21Properties of Light: Spectral Lines 1
Properties of Light: Spectral Lines 1Properties of Light: Spectral Lines 1
A description of how different chemicals can produce very specific emission lines or absorption lines in the spectrum of light from a distant object and how each chemical element has its own unique set of spectral lines. Part of an introductory series on astronomy. Let us know what you think of these videos by filling out our short survey at http://tinyurl.com/astronomy-pulsar. Thank you! -
 3:37
3:37Emission spectrum (line spectrum) - evidence for Boh'r model of an atom
Emission spectrum (line spectrum) - evidence for Boh'r model of an atomEmission spectrum (line spectrum) - evidence for Boh'r model of an atom
-
 3:41
3:41Emission and absorption line spectra
Emission and absorption line spectraEmission and absorption line spectra
By Cowen Physics (www.cowenphysics.com) In this video, Mr Cowen (@mrcowentweets) explains how emission and absorption line spectra are produced. It is aimed at GCSE students (specifically unit P7 of OCR 21st Century Science), but is also relevant to AS level physics. -
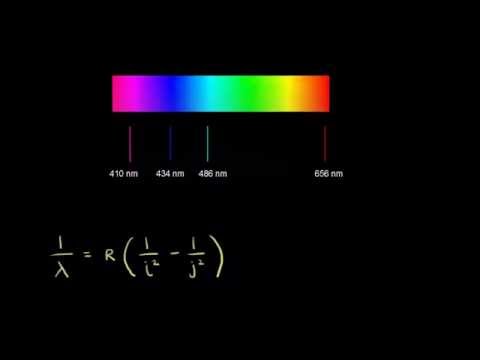 10:50
10:50Emission spectrum of hydrogen
Emission spectrum of hydrogenEmission spectrum of hydrogen
How the Balmer-Rydberg equation describes the emission spectrum of hydrogen -
 1:16
1:16Widek - Emission Line Spectrum
Widek - Emission Line SpectrumWidek - Emission Line Spectrum
Artist: Widek Song: Emission Line Spectrum Album: Aurora Borealis (EP) Country: Poland Year: 2012 Genre: Djent / Atmospheric / Progressive Metal / Instrumental WIDEK: https://www.facebook.com/WidekMusic ______________________________________ -
 6:07
6:072.2 Hydrogen emission spectrum (SL)
2.2 Hydrogen emission spectrum (SL)2.2 Hydrogen emission spectrum (SL)
This video explains the spectral lines in absorption and emission spectra, as well as the hydrogen emission spectrum. Link to worksheet with answers: https://drive.google.com/file/d/0B3Spy779s3hPSnJ0WmNtcDIzZlU/view?usp=sharing -
![C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]; updated 27 Feb 2016; published 27 Feb 2016](http://web.archive.org./web/20160704230835im_/http://i.ytimg.com/vi/oFwTPMVYfpo/0.jpg) 2:44
2:44C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]
C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]
Line spectrum - hot gas Continuous spectrum - hot source Absorption Spectrum - hot source viewed through cold gas. Thanks to Freesound.org contributors acclivity and Airborne80
- Atomic spectral line
- Atomic spectroscopy
- Atoms
- Beam emittance
- Chemical compound
- Chemical element
- Chloride
- Coefficient
- Color temperature
- Copper
- David Alter
- Diode
- Eigenstate
- Eigenvalue
- Einstein coefficient
- Electron
- Electronic structure
- Electrons
- Elementary particles
- Emission factor
- Emission lines
- Emission spectrum
- Energy
- Excited state
- Fermi's golden rule
- Flame
- Flame test
- Fluorescence
- Fraunhofer lines
- Frequencies
- Frequency
- Gamma rays
- Gamma spectroscopy
- Gustav Kirchhoff
- Hydrogen
- Ions
- Iron
- Isomeric shift
- Isotopic shift
- Light
- Luminous coefficient
- Molecular vibration
- Molecules
- Monochromatic
- Monochromator
- Natural environment
- Neon sign
- NMR spectroscopy
- Periodic table
- Perturbation theory
- Phosphorescence
- Photon
- Physics
- Planck's constant
- Planck's Constant
- Plasma (physics)
- Quantum mechanics
- Radio waves
- Raman spectroscopy
- Robert Bunsen
- Rydberg formula
- Schrödinger equation
- Sodium
- Solid
- Spectral bands
- Spectroscope
- Spectroscopy
- Spectrum analysis
- Speed of light
- Spontaneous emission
- Star
- Stefan–Boltzmann law
- Strontium
- Temperature
- Thermionic emission
- Thomson scattering
- Vibronic transition
- Visible light
- Wave function
- Wavelength
- X-ray fluorescence
- X-ray spectroscopy
-

A Level Physics - Emission and Absorption Line Spectra
Why are fireworks coloured, how can we tell what elements are in stars just from the light we see? This video explains why physics is all about sunshine and rainbows. If you would like to see more A Level Physics videos then please Subscribe to my channel to keep updated with new videos and to search the Playlists already created. You can also visit my site 'A Level Physics Online' to see how all the videos relate to your course and for even more resources at http://www.alevelphysicsonline.com/ Thanks for watching, Mr Matheson -

Emission and absorption spectra
A video explaining what emission and absorption spectra are and how they are created. This does not go into the mechanics of the Bohr atom and electron excitation. -

Emission and Absorption Spectra
086 - Emission and Absorption Spectra In this video Paul Andersen explains how the photons emitted from or absorbed by an atom or nuclei is directly related to electrons moving between energy level. Absorption and emission are a direct result of the conservation of energy. Scientists may use the emission spectra of material to determine the atoms or molecules contained within the material. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: English: Colorful Spectrum simulationEspañol: Espectro de Colores de La Luz visibleEsperanto: Koloro... -

Properties of Light: Spectral Lines 1
A description of how different chemicals can produce very specific emission lines or absorption lines in the spectrum of light from a distant object and how each chemical element has its own unique set of spectral lines. Part of an introductory series on astronomy. Let us know what you think of these videos by filling out our short survey at http://tinyurl.com/astronomy-pulsar. Thank you! -

Emission spectrum (line spectrum) - evidence for Boh'r model of an atom
-

Emission and absorption line spectra
By Cowen Physics (www.cowenphysics.com) In this video, Mr Cowen (@mrcowentweets) explains how emission and absorption line spectra are produced. It is aimed at GCSE students (specifically unit P7 of OCR 21st Century Science), but is also relevant to AS level physics. -
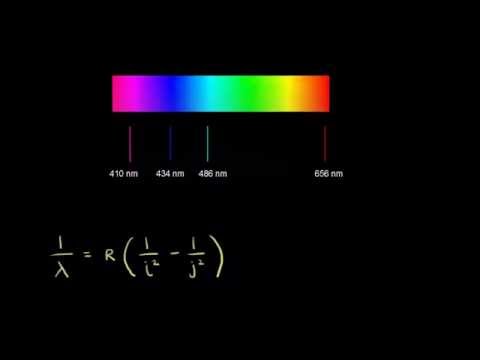
Emission spectrum of hydrogen
How the Balmer-Rydberg equation describes the emission spectrum of hydrogen -

Widek - Emission Line Spectrum
Artist: Widek Song: Emission Line Spectrum Album: Aurora Borealis (EP) Country: Poland Year: 2012 Genre: Djent / Atmospheric / Progressive Metal / Instrumental WIDEK: https://www.facebook.com/WidekMusic ______________________________________ -

2.2 Hydrogen emission spectrum (SL)
This video explains the spectral lines in absorption and emission spectra, as well as the hydrogen emission spectrum. Link to worksheet with answers: https://drive.google.com/file/d/0B3Spy779s3hPSnJ0WmNtcDIzZlU/view?usp=sharing -

C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]
Line spectrum - hot gas Continuous spectrum - hot source Absorption Spectrum - hot source viewed through cold gas. Thanks to Freesound.org contributors acclivity and Airborne80
A Level Physics - Emission and Absorption Line Spectra
- Order: Reorder
- Duration: 5:12
- Updated: 23 Feb 2015
- views: 17764
- published: 23 Feb 2015
- views: 17764
Emission and absorption spectra
- Order: Reorder
- Duration: 3:33
- Updated: 29 Apr 2013
- views: 8310
- published: 29 Apr 2013
- views: 8310
Emission and Absorption Spectra
- Order: Reorder
- Duration: 5:18
- Updated: 06 Mar 2015
- views: 58643
- published: 06 Mar 2015
- views: 58643
Properties of Light: Spectral Lines 1
- Order: Reorder
- Duration: 12:21
- Updated: 26 May 2012
- views: 47775
- published: 26 May 2012
- views: 47775
Emission spectrum (line spectrum) - evidence for Boh'r model of an atom
- Order: Reorder
- Duration: 3:37
- Updated: 10 Oct 2011
- views: 14265
- published: 10 Oct 2011
- views: 14265
Emission and absorption line spectra
- Order: Reorder
- Duration: 3:41
- Updated: 16 Nov 2014
- views: 2343
- published: 16 Nov 2014
- views: 2343
Emission spectrum of hydrogen
- Order: Reorder
- Duration: 10:50
- Updated: 23 Jun 2014
- views: 79617
- published: 23 Jun 2014
- views: 79617
Widek - Emission Line Spectrum
- Order: Reorder
- Duration: 1:16
- Updated: 02 May 2015
- views: 353
- published: 02 May 2015
- views: 353
2.2 Hydrogen emission spectrum (SL)
- Order: Reorder
- Duration: 6:07
- Updated: 05 Oct 2014
- views: 7782
- published: 05 Oct 2014
- views: 7782
C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]
- Order: Reorder
- Duration: 2:44
- Updated: 27 Feb 2016
- views: 1905
- published: 27 Feb 2016
- views: 1905
-

EEVblog #548 - EMC Pre-Compliance Conducted Emissions Testing
Dave demonstrates how to do some basic in-house EMC Pre-Compliance conducted emissions testing on a DC powered product using an open source hardware Line Impedance Stabilisation Network (LISN) box, and a low cost Rigol DSA815 spectrum analyser http://amzn.to/1b1aPJH http://www.tekbox.net/open-hardware/tboh01-5uh-impedance-stabilisation-network-lisn NOTE: As stated on the product page, this product and technique is for DC powered products only, it is not to be used for the mains supply. EMC Test Facility: http://www.youtube.com/watch?v=ZM3jWYGNoLU Forum: http://www.eevblog.com/forum/blog/eevblog-548-emc-pre-compliance-conducted-emissions-testing/ EEVblog Main Web Site: http://www.eevblog.com EEVblog Amazon Store: http://astore.amazon.com/eevblogstore-20 Donations: http://www.eevblog.com/d... -

Le Bel Air (Emission Select Tango 2/2) "Les danses la line clè"
Émission (2/2) Select Tango 1993 / RFO Partie 2 sur les danses de la "line clè' Emission sur Le Bel Air , musiques et danses ------------------------------------------------------------------- Musique : Bèlè (Biguine bèlè ou Bèlè Kourant', Bèl Dous,Bèlè Piké), Gran Bèlè, Bélia,Marine Bèlè Danses: La line Clè, Carésé, Vénézuèl, Kalenda, Kanigwé, Mabèlo, Woulé Mango Participants : Felix Caserus, Etienne Jean-Baptiste, AM4, Bel Alians, Polo Anastase, Benoit Rastocle, Georges Dru, Marie-Victoire Persani, Marie-Hélène Léotin, Jacqueline Rosemain, Féfé Marolani, Charles Philibert A VOIR AUSSI (partie 1/2) : http://youtu.be/pv56hVrSgvk -

The Fanaroff-Riley Dichotomy in AGN Jets and Their Emission-line Regions by Prajval Shastri
Extragalactic Relativistic Jets: Cause and Effect PROGRAM LINK: www.icts.res.in/program/ERG2015 DATES: Monday 12 Oct, 2015 - Tuesday 20 Oct, 2015 VENUE: Ramanujan Lecture Hall, ICTS Bangalore DESCRIPTION : Active Galactic Nuclei (AGN) are the luminous centers of galaxies that are believed to be powered by accretion of matter on to supermassive black holes. Bipolar outflows or jets that are launched from the accretion-disk black hole systems carry away angular momentum and impact the surrounding matter both inside and outside the host galaxies. Given the current computational power, AGN have become a test bed for general relativistic magnetohydrodynamics. AGN are sites of coalescing supermassive black holes, which are one of the primary science drivers of gravitational wave astronomy. ... -

Temporium - TIME LINE EMISSION DU 10 septembre 2015 - Guillaume le conquérant
"Nouvelle saison, nouvelle émission. Bienvenue dans Timeline présentée par Richard Fremder et Prem Carriou, cette semaine : Guillaume le Conquérant. Notre invité est Fabien Drugeon, réalisateur du film "Guillaume, la jeunesse du conquérant"." Cette version filmée comporte des mini-coupes toutes les 12 minutes à cause de limitations techniques. -

Atomic Emission Spectrum
Lecture on the Bohr model of the atom, atomic emission spectrum and how they apply to the real world. -

T113 Spectral lInes
Tetryonics - the charged geometry of mass-ENERGY-Matter in motion [Explaining Spectral lines, emission, absorption, quantum jumps,photo-electron] QED - Kinetic EM fields [KEM] of Matter in motion QED 29 - Absorption~Emission lines QED 30 - Lyman Spectral lines QED 31 - Balmer Spectral lines QED 32 - Paschen Spectral lines QED 33 - Brackett Spectral lines QED 34 - Pfund Spectral lines QED 35 - Humphrey Spectral lines QED 36 - Unnamed Spectral line QED 37 - The Photoelectric effect Providing the foundation for the unification of Quantum and Relativistic theories in Quantum Electrodynamics. -

Local Laboratories for high-redshift astrophysics
Speaker: Alaina Henry (GSFC) Abstract: The Lyman alpha emission line is an important diagnostic tool, used widely in attempts to understand the properties of galaxies and the intergalactic medium at early cosmic times. With a relative ease of observation at z > 2-3, and the potential to constrain outflows, dust, and neutral gas in the circumgalactic and intergalactic media, Lyman Alpha surveys have grown dramatically in the last decade. However, we are still challenged to predict Lyman alpha emission (or lack thereof); consequently, our ability to interpret high-redshift results is limited. I this talk, I will review some of the key high-redshift observations, and then turn to what we can learn from low-redshift galaxies. I will discuss new results from a sample of low-redshift obj... -

Le Bel Air (Emission Select Tango 1/2)
Emission Select Tango 1993 / RFO Emission sur Le Bel Air , musiques et danses -------------------------------------------- Musique : Bèlè (Biguine bèlè ou Bèlè Kourant', Bèl Dous,Bèlè Piké), Gran Bèlè, Bélia,Marine Bèlè Danses: La line Clè, Carésé, Vénézuèl, Kalenda, Kanigwé, Mabèlo, Woulé Mango Participants : Felix Caserus, Etienne Jean-Baptiste, AM4, Bel Alians, Polo Anastase, Benoit Rastocle, Georges Dru, Marie-Victoire Persani, Marie-Hélène Léotin, Jacqueline Rosemain, Féfé Marolani, Charles Philibert -

Purdue PHYS 342 L1.5: Classical Models: Discrete Line Spectra and Bohr’s Model
Table of Contents: 00:09 Lecture 1.5: Discrete Line Spectra and Bohr’s Model 01:13 III. Emission of Light by Atoms in Gas Discharge Tubes 04:08 Wavelengths accurately measured Why are there so many lines? 06:00 Focus on the Simplest Gas - Hydrogen 08:18 IV. Bohr Model (1911): Assumptions 10:43 Kinematical Considerations 15:07 Bohr's model for light emission from hydrogen (Bohr 1911) 16:24 Each orbit gives rise to discrete energy level 19:23 Predicting the energy levels for hydrogen This video is part of the nanoHUB hosted course "Modern Physics" (http://nanohub.org/courses/phys342) Purdue PHYS 342 provides an introduction to the physical principles underlying topics in Modern Physics. This course is intended to provide engineering undergraduate students with a firm base from which they c... -

EEVblog #548 - EMC Pre-Compliance Conducted Emissions Testing
- Order: Reorder
- Duration: 27:39
- Updated: 16 Nov 2013
- views: 43536
- published: 16 Nov 2013
- views: 43536
Le Bel Air (Emission Select Tango 2/2) "Les danses la line clè"
- Order: Reorder
- Duration: 141:36
- Updated: 21 Jan 2014
- views: 1163
- published: 21 Jan 2014
- views: 1163
The Fanaroff-Riley Dichotomy in AGN Jets and Their Emission-line Regions by Prajval Shastri
- Order: Reorder
- Duration: 75:16
- Updated: 13 Nov 2015
- views: 111
- published: 13 Nov 2015
- views: 111
Temporium - TIME LINE EMISSION DU 10 septembre 2015 - Guillaume le conquérant
- Order: Reorder
- Duration: 62:13
- Updated: 17 Sep 2015
- views: 311
- published: 17 Sep 2015
- views: 311
Atomic Emission Spectrum
- Order: Reorder
- Duration: 24:16
- Updated: 23 Oct 2012
- views: 25837
- published: 23 Oct 2012
- views: 25837
T113 Spectral lInes
- Order: Reorder
- Duration: 192:39
- Updated: 11 Jan 2013
- views: 420
- published: 11 Jan 2013
- views: 420
Local Laboratories for high-redshift astrophysics
- Order: Reorder
- Duration: 56:34
- Updated: 28 Aug 2015
- views: 147
- published: 28 Aug 2015
- views: 147
Le Bel Air (Emission Select Tango 1/2)
- Order: Reorder
- Duration: 149:03
- Updated: 07 Dec 2013
- views: 6199
- published: 07 Dec 2013
- views: 6199
Purdue PHYS 342 L1.5: Classical Models: Discrete Line Spectra and Bohr’s Model
- Order: Reorder
- Duration: 22:01
- Updated: 25 Aug 2014
- views: 316
- published: 25 Aug 2014
- views: 316
EMISSION BILAN MISS Côte d'Ivoire du 11 Juin 2016
- Order: Reorder
- Duration: 66:13
- Updated: 14 Jun 2016
- views: 3212
- Playlist
- Chat
- Playlist
- Chat

A Level Physics - Emission and Absorption Line Spectra
- Report rights infringement
- published: 23 Feb 2015
- views: 17764

Emission and absorption spectra
- Report rights infringement
- published: 29 Apr 2013
- views: 8310

Emission and Absorption Spectra
- Report rights infringement
- published: 06 Mar 2015
- views: 58643

Properties of Light: Spectral Lines 1
- Report rights infringement
- published: 26 May 2012
- views: 47775

Emission spectrum (line spectrum) - evidence for Boh'r model of an atom
- Report rights infringement
- published: 10 Oct 2011
- views: 14265

Emission and absorption line spectra
- Report rights infringement
- published: 16 Nov 2014
- views: 2343
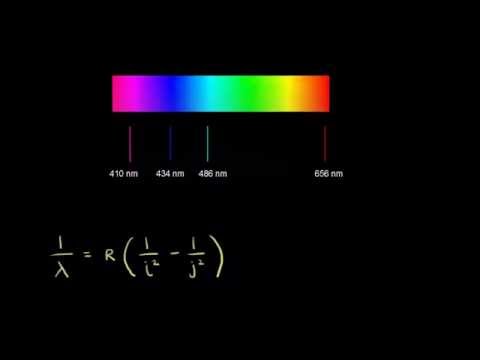
Emission spectrum of hydrogen
- Report rights infringement
- published: 23 Jun 2014
- views: 79617

Widek - Emission Line Spectrum
- Report rights infringement
- published: 02 May 2015
- views: 353

2.2 Hydrogen emission spectrum (SL)
- Report rights infringement
- published: 05 Oct 2014
- views: 7782

C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry]
- Report rights infringement
- published: 27 Feb 2016
- views: 1905
- Playlist
- Chat

EEVblog #548 - EMC Pre-Compliance Conducted Emissions Testing
- Report rights infringement
- published: 16 Nov 2013
- views: 43536

Le Bel Air (Emission Select Tango 2/2) "Les danses la line clè"
- Report rights infringement
- published: 21 Jan 2014
- views: 1163

The Fanaroff-Riley Dichotomy in AGN Jets and Their Emission-line Regions by Prajval Shastri
- Report rights infringement
- published: 13 Nov 2015
- views: 111

Temporium - TIME LINE EMISSION DU 10 septembre 2015 - Guillaume le conquérant
- Report rights infringement
- published: 17 Sep 2015
- views: 311

Atomic Emission Spectrum
- Report rights infringement
- published: 23 Oct 2012
- views: 25837

T113 Spectral lInes
- Report rights infringement
- published: 11 Jan 2013
- views: 420

Local Laboratories for high-redshift astrophysics
- Report rights infringement
- published: 28 Aug 2015
- views: 147

Le Bel Air (Emission Select Tango 1/2)
- Report rights infringement
- published: 07 Dec 2013
- views: 6199

Purdue PHYS 342 L1.5: Classical Models: Discrete Line Spectra and Bohr’s Model
- Report rights infringement
- published: 25 Aug 2014
- views: 316

EMISSION BILAN MISS Côte d'Ivoire du 11 Juin 2016
- Report rights infringement
- published: 14 Jun 2016
- views: 3212
This July 4th Americans Will Be Debating Identity Instead of Celebrating Independence
Edit WorldNews.com 04 Jul 2016Newly Disclosed US Document Shows Possible Saudi Links to 9/11 Attacks
Edit Voa News 04 Jul 2016Baghdad Bomb Toll Hits 200, Deadliest Day in a Decade
Edit NBC News 04 Jul 2016Bank of Israel buying 'hundreds of millions' of dollars: Sources
Edit The Times of India 04 Jul 2016NBA superstar Kevin Durant signs with Golden State Warriors
Edit Sydney Morning Herald 04 Jul 2016SEPTA to offer other options in response to train shortages
Edit NJ dot com 05 Jul 2016Borough Talks Series
Edit Skiddle 05 Jul 2016Deputy Mayor Visits Koinadugu District
Edit Sierra Express Media 05 Jul 2016Paul smith, Andrew Ryan, Adam Staunton & Archie Maddocks tickets
Edit Skiddle 05 Jul 2016Sneak Every Tuesdays tickets
Edit Skiddle 05 Jul 2016The first image of a new gaseous component in a planetary nebula. (IAC - Instituto de Astrofísica de Canarias)
Edit Public Technologies 04 Jul 2016BT drives for Sustainable Innovation in automotive Manufacturing and Urban Mobility (BT Group plc)
Edit Public Technologies 04 Jul 2016Shifting gears early to be ready for the future (European Union)
Edit Public Technologies 04 Jul 2016Decline in vehicular emission had no impact on pollution: CPCB
Edit The Times of India 04 Jul 2016Greener method of carbon capture using waste biomass
Edit Science Daily 04 Jul 2016York chemists lead breakthrough in carbon capture (University of York)
Edit Public Technologies 04 Jul 2016- 1
- 2
- 3
- 4
- 5
- Next page »







