- published: 16 Sep 2009
- views: 693309
-
remove the playlistInternal Energy
- remove the playlistInternal Energy
- published: 16 Feb 2015
- views: 6136
- published: 09 Sep 2010
- views: 11180
- published: 03 Oct 2014
- views: 12312
- published: 19 Sep 2013
- views: 5566
- published: 16 Sep 2009
- views: 187819
- published: 04 Oct 2012
- views: 73697

In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal energy has two major components, kinetic energy and potential energy. The kinetic energy is due to the motion of the system's particles (translations, rotations, vibrations), and the potential energy is associated with the static constituents of matter, static electric energy of atoms within molecules or crystals, and the static energy of chemical bonds. The internal energy of a system can be changed by heating the system or by doing work on it; the first law of thermodynamics states that the increase in internal energy is equal to the total heat added and work done. If the system is isolated, its internal energy cannot change.
For practical considerations in thermodynamics or engineering it is rarely necessary, nor convenient, to consider all energies belonging to the total intrinsic energy of a sample system, such as the energy given by the equivalence of mass. Typically, descriptions only include components relevant to the system under study. Thermodynamics is chiefly concerned only with changes of the internal energy.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 17:40
17:40First Law of Thermodynamics/ Internal Energy
First Law of Thermodynamics/ Internal EnergyFirst Law of Thermodynamics/ Internal Energy
First law of thermodynamic and Internal Energy More free lessons at: http://www.khanacademy.org/video?v=Xb05CaG7TsQ -
 6:15
6:15Internal Energy
Internal EnergyInternal Energy
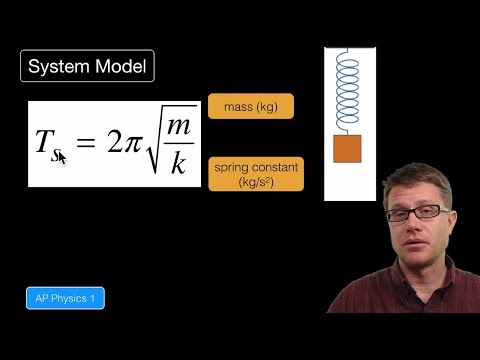
080 - Internal Energy In this video Paul Andersen explains how the internal energy of a system can change as the internal structure of the system changes. An object model will not be able to account for the restoring forces and so a system model must be used. In Physics 1 a mass-spring oscillator and simple pendulum are described. In Physics 2 a charge in an electric field is described. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: Alexandrov, Oleg. Illustration of a en:Simple Harmonic Oscillator, June 2, 2007. self-made with en:Matlab. Converted to gif animation with the en:ImageMagick convert tool (see the specific command later in the code). http://commons.wikimedia.org/wiki/File:Simple_harmonic_oscillator.gif. “Masses & Springs.” PhET. Accessed July 15, 2014. http://phet.colorado.edu/en/simulation/mass-spring-lab. “Pendulum Lab.” PhET. Accessed July 15, 2014. http://phet.colorado.edu/en/simulation/pendulum-lab. Stündle. Deutsch: Animation Einer Pendelschwingung, June 17, 2011. Own work. http://commons.wikimedia.org/wiki/File:Pendelschwingung.gif. -
 1:15
1:15Internal Energy
Internal EnergyInternal Energy
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -
 3:07
3:07Internal Energy
Internal EnergyInternal Energy
Introducing internal energy and showing a simple calculation -
 13:16
13:16First Law of Thermodynamics: Internal Energy, Heat, and Work
First Law of Thermodynamics: Internal Energy, Heat, and WorkFirst Law of Thermodynamics: Internal Energy, Heat, and Work
Lecture plus examples. Internal Energy (U or E), work, and heat is discussed. Discussion of the system and the surroundings; open and closed systems. Example calculation of work. -
 3:36
3:36Internal Energy Introduction
Internal Energy IntroductionInternal Energy Introduction
Introduces and discusses internal energy and moving boundary work on a system. Made by faculty at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Check out our Fluid Mechanics playlist: http://www.youtube.com/playlist?list=PL324604EAA66EA2F2 Check out our Thermodynamics playlists: https://www.youtube.com/user/LearnChemE/playlists?shelf_id=11&view;=50&sort;=dd Are you using a textbook? Check out our website for videos organized by topic and by chapter: http://www.learncheme.com -
 13:45
13:45More on Internal Energy
More on Internal EnergyMore on Internal Energy
Getting more intuition of internal energy, heat, and work More free lessons at: http://www.khanacademy.org/video?v=aOSlXuDO4UU -
 5:47
5:47Enthalpy H and Internal Energy U
Enthalpy H and Internal Energy U -
 1:17
1:17Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)
Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)
Can you move objects with your qi (internal energy) or slice your stomach with a Chinese cleaver without leaving a mark? Check out what this extraordinary old man in China can do! Subscribe for more Off the Great Wall: http://e.ntd.tv/SubscribeOTGW Make sure to share with your friends! Please support our show: https://subbable.com/offthegreatwall ______________________________ Want more? Check out our playlists: Viral Video Playlist: http://e.ntd.tv/ViralPlaylistOTGW OTGW Episodes Playlist: http://e.ntd.tv/EpisodesOTGW Find Off the Great Wall: Facebook: https://www.facebook.com/OffTheGreatWall Twitter: http://twitter.com/ntdotgw And let's not forget Google Plus: http://e.ntd.tv/GooglePlusOTGW Find Dan on Twitter: https://twitter.com/danotgw Find Mike on Twitter: https://twitter.com/mikexingchen Source: http://en.ntd.tv/community/newsfeed/2012/09/07/hilarious-chinese-modern-architectures-big-shorts-waist-pants-bikini/ ______________________________ MOBILE LINKS: More OTGW Vids! Online Dating for Babies: http://e.ntd.tv/WOQ646 Virtual Kissing Machine: http://e.ntd.tv/UNr1WJ Kung Fu Fighting Monkey vs. Husky Dog: http://e.ntd.tv/Y4iKfG -
 4:14
4:14Thermodynamics - Internal Energy
Thermodynamics - Internal EnergyThermodynamics - Internal Energy
Here is an introduction to internal energy. -
 2:29
2:29The Real Tai Chi Power( Internal Energy)
The Real Tai Chi Power( Internal Energy) -
 1:23
1:23What is Internal Energy?
What is Internal Energy? -
 14:08
14:08Internal Energy and Heat
Internal Energy and HeatInternal Energy and Heat
-
 2:21
2:21Relation Between Internal Energy Work And Heat
Relation Between Internal Energy Work And HeatRelation Between Internal Energy Work And Heat
Relation between internal energy, work and heat. Work:- Physical effort or activity directed towards the production or accomplishment of something is known as work. Work is a path function and not a state function. In an adiabatic process change in internal energy s Change in internal energy of a system can be done by transfer of heat from the surroundings to the system or vice-versa without expenditure of work. This results change in temperature called heat change. The transfer of heat is done through thermally conducting walls instead of adiabatic walls. In this case change in internal energy The change of state is brought about both by doing work and by transfer of heat. Change in internal energy in this case is 7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital content provider for K-12. We also customize the content as per your requirement for companies platform providers colleges etc . 7 Active driving force "The Joy of Happy Learning" -- is what makes difference from other digital content providers. We consider Student needs, Lecturer needs and College needs in designing the 3D & 2D Animated Video Lectures. We are carrying a huge 3D Digital Library ready to use. For more information: http://www.7active.in Contact: 040-64501777 / 65864777 9700061777
- Adiabatic process
- Amount of substance
- Atom
- Benjamin Thompson
- Bioenergy
- Biomass
- Brownian ratchet
- Caloric theory
- Calorimetry
- Chemical bond
- Chemical energy
- Chemical potential
- Clausius theorem
- Coal
- Cogeneration
- Compressibility
- Control volume
- Crystal
- Daniel Bernoulli
- Dipole
- Einstein notation
- Elastic energy
- Elasticity (physics)
- Electricity
- Electromagnetics
- Electrostatics
- Energetics
- Energy
- Energy carrier
- Energy level
- Ensemble average
- Enthalpy
- Entropy
- Entropy and life
- Equation of state
- Evgeny Lifshitz
- Exact differential
- Extensive quantity
- Extensive variable
- Field (physics)
- Fossil fuel
- Free entropy
- Free expansion
- Fuel oil
- Gas laws
- Georg Ernst Stahl
- Geothermal energy
- Gibbs energy
- Gibbs free energy
- Gravitation
- Heat
- Heat death paradox
- Heat engine
- Helium
- History of entropy
- History of heat
- Homogeneous function
- Hydropower
- Hydropower plant
- Ideal gas
- Ideal gas law
- Infinitesimal
- Intensive variable
- Internal energy
- Internal pressure
- Isenthalpic process
- Isentropic process
- Isobaric process
- Isochoric process
- Isolated system
- Isothermal process
- James Clerk Maxwell
- James Prescott Joule
- John James Waterston
- John Smeaton
- Josiah Willard Gibbs
- Joule
- Kilogram
- Kinetic energy
- Latent heat
- Lev Landau
- Loschmidt's paradox
- Magnetism
- Marine energy
- Mass flow
- Maxwell relation
- Maxwell relations
- Maxwell's demon
- Mean
- Mechanical work
- Mole (unit)
- Moment (physics)
- Monoatomic
- Motive power
- Natural gas
- Natural uranium
- Noble gas
- Nuclear fuel
- Nuclear power
- Nuclear power plant
- Oil refinery
- Oscillation
- Particle number
- Petroleum
- Pierre Duhem
- Polytropic process
- Potential energy
- Power (physics)
- Pressure
- Process function
- Quasistatic process
- Real gas
- Reduced properties
- Rotation
- Rudolf Clausius
- Sensible heat
- SI
- Solar energy
- Solar furnace
- Solar power
- Solar power tower
- Solar thermal energy
- State function
- State of matter
- Stress (physics)
- Synergetics (Haken)
- System
- Temperature
- Theory of heat
- Thermal efficiency
- Thermal expansion
- Thermodynamic cycle
- Thermodynamic state
- Thermodynamic system
- Thermodynamics
- Thermofluids
- Tidal power station
- Units of energy
- Vapor quality
- Vis viva
- Wave farm
- Wind energy
- Wind farm
- Wind power
- Zero point energy
-

First Law of Thermodynamics/ Internal Energy
First law of thermodynamic and Internal Energy More free lessons at: http://www.khanacademy.org/video?v=Xb05CaG7TsQ -

Internal Energy
080 - Internal Energy In this video Paul Andersen explains how the internal energy of a system can change as the internal structure of the system changes. An object model will not be able to account for the restoring forces and so a system model must be used. In Physics 1 a mass-spring oscillator and simple pendulum are described. In Physics 2 a charge in an electric field is described. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: Alexandrov, Oleg. Illustration of a en:Simple Harmonic Oscillator, June 2, 2007. self-made with en:Mat... -

Internal Energy
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -

Internal Energy
Introducing internal energy and showing a simple calculation -

First Law of Thermodynamics: Internal Energy, Heat, and Work
Lecture plus examples. Internal Energy (U or E), work, and heat is discussed. Discussion of the system and the surroundings; open and closed systems. Example calculation of work. -

Internal Energy Introduction
Introduces and discusses internal energy and moving boundary work on a system. Made by faculty at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Check out our Fluid Mechanics playlist: http://www.youtube.com/playlist?list=PL324604EAA66EA2F2 Check out our Thermodynamics playlists: https://www.youtube.com/user/LearnChemE/playlists?shelf_id=11&view;=50&sort;=dd Are you using a textbook? Check out our website for videos organized by topic and by chapter: http://www.learncheme.com -

More on Internal Energy
Getting more intuition of internal energy, heat, and work More free lessons at: http://www.khanacademy.org/video?v=aOSlXuDO4UU -

-

Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)
Can you move objects with your qi (internal energy) or slice your stomach with a Chinese cleaver without leaving a mark? Check out what this extraordinary old man in China can do! Subscribe for more Off the Great Wall: http://e.ntd.tv/SubscribeOTGW Make sure to share with your friends! Please support our show: https://subbable.com/offthegreatwall ______________________________ Want more? Check out our playlists: Viral Video Playlist: http://e.ntd.tv/ViralPlaylistOTGW OTGW Episodes Playlist: http://e.ntd.tv/EpisodesOTGW Find Off the Great Wall: Facebook: https://www.facebook.com/OffTheGreatWall Twitter: http://twitter.com/ntdotgw And let's not forget Google Plus: http://e.ntd.tv/GooglePlusOTGW Find Dan on Twitter: https://twitter.com/danotgw Find Mike on Twitter: https://twitter.... -

Thermodynamics - Internal Energy
Here is an introduction to internal energy. -

-

-

Internal Energy and Heat
-

Relation Between Internal Energy Work And Heat
Relation between internal energy, work and heat. Work:- Physical effort or activity directed towards the production or accomplishment of something is known as work. Work is a path function and not a state function. In an adiabatic process change in internal energy s Change in internal energy of a system can be done by transfer of heat from the surroundings to the system or vice-versa without expenditure of work. This results change in temperature called heat change. The transfer of heat is done through thermally conducting walls instead of adiabatic walls. In this case change in internal energy The change of state is brought about both by doing work and by transfer of heat. Change in internal energy in this case is 7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital co... -

Introduction to Breath-Control for Internal Energy, Health and Longevity
Here are 4 simple breathing exercises that can truly energise you. . This is one of the special ways that you can actually get more energy by doing less than you normally do. Natural breathing is the usually the best breath for most people to practice during most exercise. In that way you can concentrate on doing your exercise without having to worry about breathing. If, however, you are doing something very simple and relaxing such as lying down or sitting, then you can take the time to do specific breathing exercises. Essentially, there are two types of breathing exercises. One type of breathing exercise, which is commonly taught in many physical training activities including many modern yoga classes, can benefit your physical body by improving the strength and flexibility of your musc... -

Changes in Enthalpy and Internal Energy (Review)
Use the first law and the definition of enthalpy to calculate the change in internal energy and the change in enthalpy, given heat is added to a system and the volume change occurs at constant pressure. Made by faculty at the University of Colorado Boulder, Department of Chemical & Biological Engineering. Check out our chemistry playlist: http://www.youtube.com/playlist?list=PL4xAk5aclnUi1CEFNwjcheMgyWe8BwuLS -

A Level Physics - Increase in Internal Energy with Temperature
As you heat up a substance its internal energy will increase, since the particles gain more kinetic and potential energy. The temperature will also rise but not at a uniform rate. For a pure substance there will be periods where the internal energy rises but the temperature does not – as the substance changes phase from solid to liquid and again when it changes from a liquid to a gas. As it melts and boils there is a plateau in the graph (for a non-pure substance there may be a slight rise in temperature during this phase) as any energy supplied goes into breaking apart bonds rather than increasing the kinetic energy of the molecules. You can also visit my site 'A Level Physics Online' to see how all the videos relate to your course and for even more resources at http://www.alevelphysicso... -

Internal energy and temperature A2 Alevel physics revision
A recap of internal energy and temperature scales -

Chemistry Thermodynamics part 7 (Internal energy) CBSE class 11 XI
Chemistry Thermodynamics part 7 (Internal energy) CBSE class 11 XI -

ThermoChemistry & ThermoDynamics : Internal Energy - 05/42
Topics: Internal energy, Internal energy as a state function Chemistry, class XI, Unit: ThermoChemistry & ThermoDynamics Visit us at Get Full Course To Study Offline Through our website: http://www.m-learning.in Snapdeal: http://www.snapdeal.com/brand/m-learning?pageType=brandStore FlipKart: http://www.flipkart.com/computers/software/educational-media/pr?p%5B%5D=facets.brand%255B%255D%3DM%2BLearning&sid;=6bo%2C5hp%2Cvxa&ref;=78975f83-7d57-49f5-8cd3-452e26ba85bc Amazon: http://www.amazon.in/s/ref=lp_5490084031_nr_p_6_3?fst=as%3Aoff&rh;=n%3A976451031%2Cn%3A%21976452031%2Cn%3A5490084031%2Cp_6%3AA1EOND4VIENEBN&bbn;=5490084031&ie;=UTF8&qid;=1434442994&rnid;=1318474031 For More Information Call Us at :(+91) 0731-4044065, (+91)9826023096, (+91)9826023696 Mail us at :info@m-learning.in Download Stu... -

-

Thermochemistry Review P1 Internal Energy, Heat, & Work Done By The System
Thermochemistry Review: This video provides practice problems on how to calculate the change in the internal energy of a system using heat and work. It also explains the difference between what is meant by work done on the system vs work done by the system. The equations used in this video are delta E = q + w and w = -P delta V. Here is a list of problems contained in this video: 1. Calculate the change of the internal energy of a system if the system absorbs 300J of heat energy from the surroundings and performs 120J of work. 2. Determine the change of the internal energy of a system if the surroundings gain 450J of heat and performs 250J of work on the system. 3. 125J of work is done by the system. During the process, the system releases 200J of heat energy. Calculate the chang... -

First Law of Thermodynamics/ Internal Energy
- Order: Reorder
- Duration: 17:40
- Updated: 16 Sep 2009
- views: 693309
- published: 16 Sep 2009
- views: 693309
Internal Energy
- Order: Reorder
- Duration: 6:15
- Updated: 16 Feb 2015
- views: 6136
- published: 16 Feb 2015
- views: 6136
Internal Energy
- Order: Reorder
- Duration: 1:15
- Updated: 09 Sep 2010
- views: 11180
- published: 09 Sep 2010
- views: 11180
Internal Energy
- Order: Reorder
- Duration: 3:07
- Updated: 18 Sep 2012
- views: 4735
First Law of Thermodynamics: Internal Energy, Heat, and Work
- Order: Reorder
- Duration: 13:16
- Updated: 03 Oct 2014
- views: 12312
- published: 03 Oct 2014
- views: 12312
Internal Energy Introduction
- Order: Reorder
- Duration: 3:36
- Updated: 19 Sep 2013
- views: 5566
- published: 19 Sep 2013
- views: 5566
More on Internal Energy
- Order: Reorder
- Duration: 13:45
- Updated: 16 Sep 2009
- views: 187819
- published: 16 Sep 2009
- views: 187819
Enthalpy H and Internal Energy U
- Order: Reorder
- Duration: 5:47
- Updated: 03 Oct 2014
- views: 4483
Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)
- Order: Reorder
- Duration: 1:17
- Updated: 04 Oct 2012
- views: 73697
- published: 04 Oct 2012
- views: 73697
Thermodynamics - Internal Energy
- Order: Reorder
- Duration: 4:14
- Updated: 22 Jun 2012
- views: 6114
- published: 22 Jun 2012
- views: 6114
The Real Tai Chi Power( Internal Energy)
- Order: Reorder
- Duration: 2:29
- Updated: 11 Aug 2013
- views: 9611
What is Internal Energy?
- Order: Reorder
- Duration: 1:23
- Updated: 09 Jul 2012
- views: 1439
Internal Energy and Heat
- Order: Reorder
- Duration: 14:08
- Updated: 29 Nov 2011
- views: 3099
- published: 29 Nov 2011
- views: 3099
Relation Between Internal Energy Work And Heat
- Order: Reorder
- Duration: 2:21
- Updated: 09 May 2014
- views: 2845
- published: 09 May 2014
- views: 2845
Introduction to Breath-Control for Internal Energy, Health and Longevity
- Order: Reorder
- Duration: 4:40
- Updated: 30 Sep 2015
- views: 1806
- published: 30 Sep 2015
- views: 1806
Changes in Enthalpy and Internal Energy (Review)
- Order: Reorder
- Duration: 2:57
- Updated: 22 Jan 2014
- views: 5376
- published: 22 Jan 2014
- views: 5376
A Level Physics - Increase in Internal Energy with Temperature
- Order: Reorder
- Duration: 3:01
- Updated: 28 Nov 2015
- views: 382
- published: 28 Nov 2015
- views: 382
Internal energy and temperature A2 Alevel physics revision
- Order: Reorder
- Duration: 14:27
- Updated: 28 Apr 2015
- views: 472
- published: 28 Apr 2015
- views: 472
Chemistry Thermodynamics part 7 (Internal energy) CBSE class 11 XI
- Order: Reorder
- Duration: 10:40
- Updated: 13 Feb 2013
- views: 18042
- published: 13 Feb 2013
- views: 18042
ThermoChemistry & ThermoDynamics : Internal Energy - 05/42
- Order: Reorder
- Duration: 20:44
- Updated: 23 Oct 2013
- views: 6728
- published: 23 Oct 2013
- views: 6728
Physics - Thermodynamics: (2 of 22) Internal Energy Of A Gas
- Order: Reorder
- Duration: 8:09
- Updated: 17 Jul 2013
- views: 31654
Thermochemistry Review P1 Internal Energy, Heat, & Work Done By The System
- Order: Reorder
- Duration: 11:19
- Updated: 15 Nov 2015
- views: 342
- published: 15 Nov 2015
- views: 342
Calculate Total Change In Internal Energy (∆E) Using Heat and WorkPV 002
- Order: Reorder
- Duration: 5:36
- Updated: 13 Nov 2012
- views: 7210
- Playlist
- Chat
- Playlist
- Chat

First Law of Thermodynamics/ Internal Energy
- Report rights infringement
- published: 16 Sep 2009
- views: 693309

Internal Energy
- Report rights infringement
- published: 16 Feb 2015
- views: 6136

Internal Energy
- Report rights infringement
- published: 09 Sep 2010
- views: 11180

Internal Energy
- Report rights infringement
- published: 18 Sep 2012
- views: 4735

First Law of Thermodynamics: Internal Energy, Heat, and Work
- Report rights infringement
- published: 03 Oct 2014
- views: 12312

Internal Energy Introduction
- Report rights infringement
- published: 19 Sep 2013
- views: 5566

More on Internal Energy
- Report rights infringement
- published: 16 Sep 2009
- views: 187819

Enthalpy H and Internal Energy U
- Report rights infringement
- published: 03 Oct 2014
- views: 4483

Amazing Old Man Moves Bricks and Breaks Glass with Qi (Internal Energy)
- Report rights infringement
- published: 04 Oct 2012
- views: 73697

Thermodynamics - Internal Energy
- Report rights infringement
- published: 22 Jun 2012
- views: 6114

The Real Tai Chi Power( Internal Energy)
- Report rights infringement
- published: 11 Aug 2013
- views: 9611

What is Internal Energy?
- Report rights infringement
- published: 09 Jul 2012
- views: 1439

Internal Energy and Heat
- Report rights infringement
- published: 29 Nov 2011
- views: 3099

Relation Between Internal Energy Work And Heat
- Report rights infringement
- published: 09 May 2014
- views: 2845
New Study Suggests Bible Could Have Been Written Earlier Than Previously Thought
Edit WorldNews.com 12 Apr 2016NASA Scientists Regain Control Of Kepler, Trying To Figure Out Outage Cause
Edit WorldNews.com 11 Apr 2016CBS’ ‘60 Minutes’ Vindicates Trump But Republican Purge Continues
Edit WorldNews.com 11 Apr 2016Egypt gives Saudi Arabia 2 islands in a show of gratitude
Edit The Times of India 11 Apr 2016Immigrant students blocked from enrolling in school in the US: Report
Edit Deccan Herald 11 Apr 2016Gas industry leaders go into battle for LNG
Edit Sydney Morning Herald 11 Apr 2016Oil rises on signs of tightening market, but economic worries weigh
Edit The Times of India 11 Apr 201670-90% Decline In Well Completions Raises Hope For Oil & Gas
Edit Oil Price 11 Apr 2016Iraq Boosts Oil Production to Record Before Talks to Cap Output
Edit Bloomberg 10 Apr 2016Fuel queues back with a vengeance stalling Nigerian economy
Edit The Times of India 10 Apr 2016Can We Now Predict When A Neutron Star Will Give Birth To A Black Hole?
Edit Universe Today 10 Apr 2016China, Japan, U.S. leading solar energy boom
Edit Houston Chronicle 08 Apr 2016Iran Plans to Add One Qatar to Oil Supply as Others Seek Freeze
Edit Topix 08 Apr 2016Oil Market `Fooled' by Freeze Talks Seen Better Off Gauging U.S.
Edit Bloomberg 08 Apr 2016IEA Executive Director discusses opportunities and challenges in Thailand’s energy sector with Deputy Prime Minister of Thailand (IEA - International Energy Agency)
Edit Public Technologies 07 Apr 2016COP21 goals are the challenge for Slovenian energy policy (WEC - World Energy Council)
Edit Public Technologies 07 Apr 2016GOVERNOR KASICH FAILS TO ARTICULATE ENERGY VISION FOR OHIO (Sierra Club)
Edit Public Technologies 07 Apr 2016Issues important for Lithuania's energy security discussed in Washington, DC (Ministry of Foreign Affairs of the Republic of Lithuania)
Edit Public Technologies 07 Apr 2016- 1
- 2
- 3
- 4
- 5
- Next page »







