- published: 27 Jul 2009
- views: 46793
-
remove the playlistAtomic Nuclei
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistAtomic Nuclei
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 04 May 2014
- views: 22330
- published: 10 Sep 2010
- views: 10019
- published: 01 Mar 2008
- views: 273684
- published: 12 Feb 2013
- views: 1990036
- published: 17 Feb 2012
- views: 39287
- published: 10 Jan 2014
- views: 2306
- published: 03 Sep 2014
- views: 244
- published: 16 Apr 2012
- views: 1768950
- published: 13 Mar 2016
- views: 4

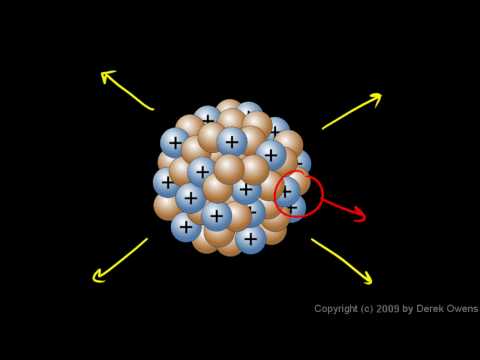
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932.[citation needed] Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the orbiting electrons.
The diameter of the nucleus is in the range of 1.75 fm (femtometre) (1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 6:27
6:27Physical Science 7.4c - The Atomic Nucleus
Physical Science 7.4c - The Atomic NucleusPhysical Science 7.4c - The Atomic Nucleus
From the Physical Science course by Derek Owens. Eighth grade level. Distance Learning courses are available at http://www.derekowens.com -
 7:04
7:04Atomic Nucleus
Atomic NucleusAtomic Nucleus
003 - Atomic Nucleus In this video Paul Andersen explains how the structure of the nucleus influences the properties of the atom. The number of the protons determines the kind of element. Isotopes are formed when the number of protons remain the same but the neutrons are different. Some isotopes are radioactive and may decay over time. The rate of decay is the half-life and can be used to measure decay or time. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2008, Drawn by User:Fastfission in Illustrator and Inkscape--Fastfission 15:04, 14 April. English: Top: Expected Results of Rutherford's Gold Foil Experiment: Alpha Particles Passing through the Plum Pudding Model of the Atom Undisturbed. Bottom: Observed Results: Some of the Particles Were Deflected, and Some by Very Large Angles. Rutherford Concluded That the Positive Charge of the Atom Must Be Concentrated into a Very Small Location: The Atomic Nucleus., April 2008. Own work. http://commons.wikimedia.org/wiki/File:Rutherford_gold_foil_experiment_results.svg. "File:Alfa Beta Gamma Radiation.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Alfa_beta_gamma_radiation.svg. "File:Alpha Decay.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Alpha_Decay.svg. "File:Ernest Rutherford Cropped.jpg." Wikipedia, the Free Encyclopedia. Accessed April 26, 2014. http://en.wikipedia.org/wiki/File:Ernest_Rutherford_cropped.jpg. "File:Nucleus Drawing.svg." Wikipedia, the Free Encyclopedia. Accessed April 26, 2014. http://en.wikipedia.org/wiki/File:Nucleus_drawing.svg. File:Plum Pudding Atom.svg, n.d. http://commons.wikimedia.org/wiki/File:Plum_pudding_atom.svg. "File:Table Isotopes En.svg." Wikipedia, the Free Encyclopedia. Accessed April 27, 2014. http://en.wikipedia.org/wiki/File:Table_isotopes_en.svg. LeVanHan. English: Periodic Table of the Elements, 2008. Own work. http://commons.wikimedia.org/wiki/File:Periodic-table.jpg. London, Jay Springett from. English: Christmas Pudding with Flaming Rum, December 1, 2011. rum This photograph was submitted to Wikimedia Commons by "Will Murray (Willscrlt)". http://commons.wikimedia.org/wiki/File:Christmas_Pudding_with_Flaming_Rum.jpg. Rosenkrantz, Kurt. English: Decay of an Imaginary Radioactive Substance with a Half-Life of One Year., July 27, 2010. http://cafreetextbooks.ck12.org/science/CK12_Earth_Science_rev.pdf (page 433) If the above link no longer works, visit http://www.ck12.org and search for CK-12 Earth Science. http://commons.wikimedia.org/wiki/File:Radioactive_decay.png. -
 8:54
8:54Atomic Nucleus
Atomic NucleusAtomic Nucleus
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -
 3:28
3:28The Discovery of the Atomic Nucleus (3 of 15)
The Discovery of the Atomic Nucleus (3 of 15)The Discovery of the Atomic Nucleus (3 of 15)
Episode 3 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.com/STFC_Matters Facebook: https://facebook.com/SciTechFacCouncil LinkedIn: https://www.linkedin.com/company/stfc -
 10:12
10:12The Nucleus: Crash Course Chemistry #1
The Nucleus: Crash Course Chemistry #1The Nucleus: Crash Course Chemistry #1
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is. Like CrashCourse? http://www.facebook.com/YouTubeCrashCourse Follow CrashCourse! http://www.twitter.com/TheCrashCourse Tumbl CrashCourse. http://thecrashcourse.tumblr.com Table of Contents Einstein & Atoms 02:05 Composition of Atoms 03:18 Atomic Number 04:20 Isotopes 08:04 Relative Atomic Mass 07:26 Mass Number 07:44 Watch the SciShow episodes on the Strong Nuclear Force here: http://www.youtube.com/watch?v=Yv3EMq2Dgq8 and http://www.youtube.com/watch?v=BNDOSMqGLlg Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 8:35
8:35Baths and Quarks: Solitons explained
Baths and Quarks: Solitons explainedBaths and Quarks: Solitons explained
In 'Baths and Quarks', theoretical physics expert David Tong explains solitons and their effect on quarks and protons. 'Solitons' -- solitary waves which can be seen as bubble rings in the bath -- make it impossible for quarks and protons to be separated, thus holding together the universe, he says. "Baths would be so much more relaxing if they weren't so interesting. Bubble rings - there's something strange and unnatural about these objects - so structural where you wouldn't expect to see structure. When I get out of the bath and pull the plug, there's a world of water that drains away - a vortex - it's very similar to the bubble rings, and objects like these may just hold the key to one of the most important problems in particle physics [relating to quarks and protons]. My name is David Tong. My job is to understand the beautiful things that I see in the world around me. But to describe them properly, I have to understand them in the language in which nature is written." -
 4:58
4:5839 1 The Atomic Nucleus and Radioactivity
39 1 The Atomic Nucleus and Radioactivity39 1 The Atomic Nucleus and Radioactivity
-
 4:01
4:01Neutron stars - A melting pot for atomic nuclei
Neutron stars - A melting pot for atomic nucleiNeutron stars - A melting pot for atomic nuclei
CC-BY-ND Neutron stars are extraordinary objects in the universe. Their density is so high that atoms melt within them and new states of matter arise. Tetyana Galatyuk and her colleagues try to simulate these conditions at the smallest scale using the FAIR particle accelerator and observe the results with the CBM detector. Thus, they not only learn more about neutron stars, but also about the innermost structure of matter. Tetyana Galatyuk is head of a junior research group at GSI Helmholtz and the TU Darmstadt. With her group, she carries out experiments using HADES and CBM to explore compressed nuclear matter. Galatyuk studied physics in Ukraine, worked with the STAR detector at the RHIC accelerator facility in the U.S. and subsequently conducted research in Germany. -
 59:11
59:11XII-10.1.Atom Introduction (2014)Pradeep Kshetrapal Physics
XII-10.1.Atom Introduction (2014)Pradeep Kshetrapal Physics -
 17:35
17:35Collisions of Elementary Particles with Atomic Nuclei - Roy Glauber
Collisions of Elementary Particles with Atomic Nuclei - Roy GlauberCollisions of Elementary Particles with Atomic Nuclei - Roy Glauber
Source - http://serious-science.org/videos/1197 Nobel Prize laureate Roy Glauber on Rutherford’s experiments, exploring uranium fission products, and development of the nuclear weapon -
 5:28
5:28Just How Small is an Atom?
Just How Small is an Atom?Just How Small is an Atom?
Just how small are atoms? And what's inside them? The answers turn out to be astounding, even for those who think they know. This fast-paced animation uses spectacular metaphors (imagine a blueberry the size of a football stadium!) to give a visceral sense of the building blocks that make our world. Lesson by Jonathan Bergmann, animation by Cognitive Media. -
 2:44
2:44Ferry Cross the Mersey - played with atomic nuclei
Ferry Cross the Mersey - played with atomic nucleiFerry Cross the Mersey - played with atomic nuclei
"Gerry and the Pacemakers" were a very famous Liverpool group in the 1960ies. One of their greatest songs was "Ferry Cross the Mersey", a beautiful melody. The recording in 1964 has been produced by the recently deceased Sir George Martin. Here, you'll be hearing this song played by using the 1H-atomic nuclei of acetone, a simple organic molecule. The magnetic properties of this molecule are usually exploited in a Nuclear Magnetic Resonance spectrometer (NMR). However, by some hardware modifications, it's possible to make the atomic nuclei audible. This is done here, and my youth favourite song is combined with my profession (NMR spectroscopist). For more information, please Google "Walter Bauer Erlangen NMR". -
 5:53
5:53Designer Atomic Nuclei: Interview with NSCL's Brad Sherrill
Designer Atomic Nuclei: Interview with NSCL's Brad SherrillDesigner Atomic Nuclei: Interview with NSCL's Brad Sherrill
Brad Sherrill, NSCL Associate Director for Research, discusses his May 9, 2008 Perspective in the journal Science: "Designer Atomic Nuclei -
 3:33
3:33Against All Odds - sounds from atomic nuclei
Against All Odds - sounds from atomic nucleiAgainst All Odds - sounds from atomic nuclei
This is a copy of a movie trailer by Phil Collins that has been released on YouTube a couple of years ago. However, any sounds come (lip sync) from the 1H-atomic nuclei of acetone, based on their Nuclear Magnetic Resonance (NMR) properties. For more information, please Google "Walter Bauer Erlangen NMR".
- Activation product
- Atom
- Atomic mass
- Atomic nucleus
- Atomic number
- Atomic orbital
- Atomic physics
- Axino
- Axion
- B meson
- Baryon
- Binding energy
- Bismuth-209
- Book Hadronic Matter
- Book Leptons
- Book Quarks
- Boron-14
- Borromean rings
- Boson
- Bottom antiquark
- Bottom quark
- Bound state
- Brachytherapy
- Bubble fusion
- CANDU reactor
- Chargino
- Charm antiquark
- Charm quark
- Chemical element
- D meson
- Davydov soliton
- Delta baryon
- Dense plasma focus
- Depleted uranium
- Deuterium
- Deuteron
- Dibaryon
- Dilaton
- Dineutron
- Diquark
- Dmitry Ivanenko
- Down antiquark
- Down quark
- Dry cask storage
- Electron
- Electron cloud
- Electron hole
- Electron neutrino
- Elementary particle
- Enriched uranium
- Ernest Marsden
- Ernest Rutherford
- Eta meson
- Eta prime meson
- Exciton
- Exotic atom
- Exotic baryon
- Exotic hadron
- Exotic meson
- Faddeev–Popov ghost
- Fast breeder reactor
- Fast neutron
- Fast-neutron reactor
- Femtometre
- Fermion
- Fertile material
- Fissile
- FLiBe
- Fusion neutron
- Fusion power
- Fusor
- Gamma camera
- Gauge boson
- Gaugino
- Giant resonance
- Gilbert N. Lewis
- Glueball
- Gluino
- Gluon
- Gravitino
- Graviton
- Hadron
- Halo nucleus
- Hans Geiger
- Heavy water reactor
- Helium
- Helium-3
- Helium-4
- Higgs boson
- Higgsino
- High level waste
- Hydrogen
- Hydrogen-2
- Hydrogen-3
- Hypernucleus
- Hyperon
- Ionizing radiation
- Isospin
- Isotope
- Isotope separation
- J ψ meson
- John Wiley & Sons
- Kaon
- Kluwer Academic
- Lambda baryon
- Lead-208
- Lepton
- Levitated dipole
- Light water reactor
- Liquid drop model
- Liquid helium
- Liquid-drop model
- List of baryons
- List of mesons
- List of particles
- Lithium-11
- Lithium-6
- Low level waste
- Magnetic monopole
- Magnon
- Magnox
- Majorana fermion
- Majoron
- Mass number
- Medical imaging
- Meson
- Mesonic molecule
- Metre
- Michael Faraday
- Migma
- Minor actinide
- Molecule
- Molten salt reactor
- Muon
- Muon neutrino
- Muonium
- Neutralino
- Neutron
- Neutron activation
- Neutron capture
- Neutron generator
- Neutron moderator
- Neutron poison
- Neutron radiation
- Neutron reflector
- Neutron temperature
- Nuclear arms race
- Nuclear chemistry
- Nuclear engineering
- Nuclear explosion
- Nuclear fission
- Nuclear force
- Nuclear fuel
- Nuclear fuel cycle
- Nuclear fusion
- Nuclear medicine
- Nuclear physics
- Nuclear power
- Nuclear power debate
- Nuclear power plant
- Nuclear propulsion
- Nuclear reactor
- Nuclear reprocessing
- Nuclear safety
- Nuclear shell model
- Nuclear size
- Nuclear structure
- Nuclear technology
- Nuclear warfare
- Nuclear weapon
- Nuclear weapon yield
- Nucleon
- Omega baryon
- Omega meson
- Onium
- Particle physics
- Pebble bed reactor
- Pentaquark
- Phi meson
- Phonon
- Photon
- Pion
- Plasmaron
- Plasmon
- Plum pudding model
- Plutonium
- Polariton
- Polaron
- Polywell
- Pomeron
- Positron
- Positronium
- Prolate
- Promethium
- Proton
- Proton therapy
- Pyroelectric fusion
- Quantum number
- Quark
- Quarkonium
- Quasiparticle
- Radiation
- Radiation therapy
- Radioactive waste
- Radioactivity
- RBMK
- Reprocessed uranium
- Reversed field pinch
- Rho meson
- Roton
- Rutherford model
- Schrödinger
- Scintigraphy
- Sfermion
- SGHWR
- Sigma baryon
- Skyrmion
- Spent fuel pool
- Spent nuclear fuel
- Spheromak
- Springer (publisher)
- SSTAR
- Stellarator
- Sterile neutrino
- Strange antiquark
- Strange quark
- Strangeness
- Strong interaction
- Superatom
- Superfluid
- Superpartner
- T meson
- Tachyon
- Talk Atomic nucleus
- Tau (particle)
- Tau neutrino
- Tauonium
- Technetium
- Template Particles
- Tetraquark
- Thermal neutron
- Theta meson
- Thorium
- Tin
- TNT equivalent
- Tokamak
- Tomotherapy
- Top antiquark
- Top quark
- Trion (physics)
- Tritium
- UHTREX
- Up antiquark
- Up quark
- Upsilon meson
- Uranium
- Van der Waals force
- VVER
- W and Z bosons
- W' and Z' bosons
- Wave function
- Wikipedia Books
- X and Y bosons
- X-ray
- Xi baryon
- Yukawa potential
- Z-pinch
-

Physical Science 7.4c - The Atomic Nucleus
From the Physical Science course by Derek Owens. Eighth grade level. Distance Learning courses are available at http://www.derekowens.com -

Atomic Nucleus
003 - Atomic Nucleus In this video Paul Andersen explains how the structure of the nucleus influences the properties of the atom. The number of the protons determines the kind of element. Isotopes are formed when the number of protons remain the same but the neutrons are different. Some isotopes are radioactive and may decay over time. The rate of decay is the half-life and can be used to measure decay or time. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: 2008, Drawn by User:Fastfission in Illustrator and Inkscape--Fastfission 15:... -

Atomic Nucleus
Watch more videos on http://www.brightstorm.com/science/physics SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► https://www.facebook.com/brightstorm Pinterest ► https://www.pinterest.com/brightstorm/ Google+ ► https://plus.google.com/+brightstorm/ Twitter ► https://twitter.com/brightstorm_ Brightstorm website ► https://www.brightstorm.com/ -

The Discovery of the Atomic Nucleus (3 of 15)
Episode 3 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.co... -

The Nucleus: Crash Course Chemistry #1
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is. Like CrashCourse? http://www.facebook.com/YouTubeCrashCourse Follow CrashCourse! http://www.twitter.com/TheCrashCourse Tumbl CrashCourse. http://thecrashcourse.tumblr.com Table of Contents Einstein & Atoms 02:05 Composition of Atoms 03:18 Atomic Num... -

Baths and Quarks: Solitons explained
In 'Baths and Quarks', theoretical physics expert David Tong explains solitons and their effect on quarks and protons. 'Solitons' -- solitary waves which can be seen as bubble rings in the bath -- make it impossible for quarks and protons to be separated, thus holding together the universe, he says. "Baths would be so much more relaxing if they weren't so interesting. Bubble rings - there's something strange and unnatural about these objects - so structural where you wouldn't expect to see structure. When I get out of the bath and pull the plug, there's a world of water that drains away - a vortex - it's very similar to the bubble rings, and objects like these may just hold the key to one of the most important problems in particle physics [relating to quarks and protons]. My name is David ... -

39 1 The Atomic Nucleus and Radioactivity
-

Neutron stars - A melting pot for atomic nuclei
CC-BY-ND Neutron stars are extraordinary objects in the universe. Their density is so high that atoms melt within them and new states of matter arise. Tetyana Galatyuk and her colleagues try to simulate these conditions at the smallest scale using the FAIR particle accelerator and observe the results with the CBM detector. Thus, they not only learn more about neutron stars, but also about the innermost structure of matter. Tetyana Galatyuk is head of a junior research group at GSI Helmholtz and the TU Darmstadt. With her group, she carries out experiments using HADES and CBM to explore compressed nuclear matter. Galatyuk studied physics in Ukraine, worked with the STAR detector at the RHIC accelerator facility in the U.S. and subsequently conducted research in Germany. -

-

Collisions of Elementary Particles with Atomic Nuclei - Roy Glauber
Source - http://serious-science.org/videos/1197 Nobel Prize laureate Roy Glauber on Rutherford’s experiments, exploring uranium fission products, and development of the nuclear weapon -

Just How Small is an Atom?
Just how small are atoms? And what's inside them? The answers turn out to be astounding, even for those who think they know. This fast-paced animation uses spectacular metaphors (imagine a blueberry the size of a football stadium!) to give a visceral sense of the building blocks that make our world. Lesson by Jonathan Bergmann, animation by Cognitive Media. -

Ferry Cross the Mersey - played with atomic nuclei
"Gerry and the Pacemakers" were a very famous Liverpool group in the 1960ies. One of their greatest songs was "Ferry Cross the Mersey", a beautiful melody. The recording in 1964 has been produced by the recently deceased Sir George Martin. Here, you'll be hearing this song played by using the 1H-atomic nuclei of acetone, a simple organic molecule. The magnetic properties of this molecule are usually exploited in a Nuclear Magnetic Resonance spectrometer (NMR). However, by some hardware modifications, it's possible to make the atomic nuclei audible. This is done here, and my youth favourite song is combined with my profession (NMR spectroscopist). For more information, please Google "Walter Bauer Erlangen NMR". -

Designer Atomic Nuclei: Interview with NSCL's Brad Sherrill
Brad Sherrill, NSCL Associate Director for Research, discusses his May 9, 2008 Perspective in the journal Science: "Designer Atomic Nuclei -

Against All Odds - sounds from atomic nuclei
This is a copy of a movie trailer by Phil Collins that has been released on YouTube a couple of years ago. However, any sounds come (lip sync) from the 1H-atomic nuclei of acetone, based on their Nuclear Magnetic Resonance (NMR) properties. For more information, please Google "Walter Bauer Erlangen NMR". -

Hello - song by Lionel Richie played with atomic nuclei
"Hello" is a song by Lionel Richie, released in 1984 (not to be confused with the same name song by Adele of 2015!). A very romantic song, and I've tried to do something special: playing this song by using the sound of atomic nuclei. Any tones you'll be hearing (including bass, drums etc.) originate from the 1H-atomic nuclei of acetone, a very simple organic molecule. The atomic nuclei can be excited in a Nuclear Magnetic Resonance Spectrometer (NMR) which is normally used for spectral analysis of unknown molecules. However, some modificatiions make the nuclei audible, and by superimposing various tracks this melody is produced. For more information, please Google "Walter Bauer Erlangen NMR". -

FROM ATOMIC NUCLEI TO STARS!
平成26年度理学部ティータイムセミナー 講師:理化学研究所 SARAH NAIMI 氏 日時:平成26年7月15日(火) 16:20~17:50 場所:埼玉大学理学部講義実験棟1F 4番教室 【概要】第4回ティータイムセミナーでは、理化学研究所で加速器物理学を研究されている、アルジェリアーフランスからの女性研究者を囲んで、研究活動のこと、出身国のお話、日本での生活での楽しみや?なことなど、ざっくばらんにお話して頂き、参加者がそれに交わっていくことを期待しています。理学部学科を問わず、お時間があればお気軽にご参加ください。 -

Half Moon Bay - song by Dan Fogelberg played with atomic nuclei
Dan Fogelberg was an ingenious singer and songwriter - my absolute personal hero. Sadly, he deceased in 2007. From his 2003 CD "Full Circle", here's the overture "Half Moon Bay", one of the most beautiful melodies ever heard. I've played this song by using the 1H-atomic nuclei of acetone, a simple organic molecule. The tones have been generated in a Nuclear Magnetic Resonance (NMR) spectrometer by exploting the magnetic properties of the acetone protons. For more information, please Google "Walter Bauer Erlangen NMR". -

Dr Shivago - movie title song played by atomic nuclei
"Dr. Shivago" is a very famous movie, released in 1965. Famous actors like Omar Sharif and Julie Christie. The movie title melody is known to everyone. Here, you'll be hearing this melody played on a Nuclear Magnetic Resonance (NMR) spectrometer by exploiting the 1H-nuclear properties of acetone, a very simple molecule. For more information, please Google "Walter Bauer Erlangen NMR". -

Formation of the Atomic Nuclei
Geometric Quantisation and presentation of Ground State Degeneracy,Skyrmion resonance modes Composite Fermions and the formation of the atomic structure. -

Photoelectric Effect | Modern Physics (Atoms, Nuclei)
"Link to Buy: -http://goo.gl/EhskZu Mohammed Aziz Alam (IIT Roorkee) in this video explains the feud between the two sections of scientific community regarding the question of nature of light. He then introduces the experiment of Photoelectric Effect (Einstein''s Idea) which explains the light as a matter. This experiment was a major step towards understanding the nature of light because it affirms the light as matter which is in total contrast to Young''s Experiment proving light as a wave. Chapter Name -Modern Physics (Atoms, Nuclei) Prepared by - Aziz Alam, IIT Roorkee, 4 Years of Exp., Physics This chapter covers Introduction to Modern Physics, Photoelectric Effect, Introduction of Modern Physics, de Broglie's Hypothesis, Plum-Pudding Model and Rutherford's Model, X-Rays, Bohr's Atom... -

Forces in atomic nuclei (with subtitles)
An extract from the lecture "A journey to microworld", lecture about matter and energy, elementary particles and forces in the nature. In this 9-minute extract, Jovan Aleksic shows how atomic nuclei are built and which forces are responsible for this. -

Teach Astronomy - Energy from Atomic Nuclei
http://www.teachastronomy.com/ Physicists in the nineteenth century made various estimates of the age of the Sun, but they were fundamentally unaware of the most efficient energy source known. Early in the twentieth century physicists Rutherford and Becquerel began a systematic study of the phenomenon of radioactivity, a situation where atoms spontaneously emit both particles and radiation. Rutherford for example sealed a small amount of a radioactive substance in a tube that contained a pure vacuum. He returned months later to find that the tube contained helium gas and that the chemical properties of the radioactive substance had changed. Here was proof both that the atomic nucleus can emit energy and that chemicals can change fundamentally due to radioactive processes. The atomic n... -

Chemistry: The Stability of Atomic Nuclei
http://www.mindbites.com/lesson/4836 for full video. Check out http://www.mindbites.com/series/549-chemistry-full-course for a comprehensive video-based chemistry course or http://www.mindbites.com/category/24-chemistry for additional individual video lessons on specific Chemistry concepts and topics of study.
Physical Science 7.4c - The Atomic Nucleus
- Order: Reorder
- Duration: 6:27
- Updated: 27 Jul 2009
- views: 46793
- published: 27 Jul 2009
- views: 46793
Atomic Nucleus
- Order: Reorder
- Duration: 7:04
- Updated: 04 May 2014
- views: 22330
- published: 04 May 2014
- views: 22330
Atomic Nucleus
- Order: Reorder
- Duration: 8:54
- Updated: 10 Sep 2010
- views: 10019
- published: 10 Sep 2010
- views: 10019
The Discovery of the Atomic Nucleus (3 of 15)
- Order: Reorder
- Duration: 3:28
- Updated: 01 Mar 2008
- views: 273684
- published: 01 Mar 2008
- views: 273684
The Nucleus: Crash Course Chemistry #1
- Order: Reorder
- Duration: 10:12
- Updated: 12 Feb 2013
- views: 1990036
- published: 12 Feb 2013
- views: 1990036
Baths and Quarks: Solitons explained
- Order: Reorder
- Duration: 8:35
- Updated: 17 Feb 2012
- views: 39287
- published: 17 Feb 2012
- views: 39287
39 1 The Atomic Nucleus and Radioactivity
- Order: Reorder
- Duration: 4:58
- Updated: 20 Mar 2014
- views: 1576
- published: 20 Mar 2014
- views: 1576
Neutron stars - A melting pot for atomic nuclei
- Order: Reorder
- Duration: 4:01
- Updated: 10 Jan 2014
- views: 2306
- published: 10 Jan 2014
- views: 2306
XII-10.1.Atom Introduction (2014)Pradeep Kshetrapal Physics
- Order: Reorder
- Duration: 59:11
- Updated: 23 Nov 2014
- views: 11931
Collisions of Elementary Particles with Atomic Nuclei - Roy Glauber
- Order: Reorder
- Duration: 17:35
- Updated: 03 Sep 2014
- views: 244
- published: 03 Sep 2014
- views: 244
Just How Small is an Atom?
- Order: Reorder
- Duration: 5:28
- Updated: 16 Apr 2012
- views: 1768950
- published: 16 Apr 2012
- views: 1768950
Ferry Cross the Mersey - played with atomic nuclei
- Order: Reorder
- Duration: 2:44
- Updated: 13 Mar 2016
- views: 4
- published: 13 Mar 2016
- views: 4
Designer Atomic Nuclei: Interview with NSCL's Brad Sherrill
- Order: Reorder
- Duration: 5:53
- Updated: 09 May 2008
- views: 633
- published: 09 May 2008
- views: 633
Against All Odds - sounds from atomic nuclei
- Order: Reorder
- Duration: 3:33
- Updated: 12 Mar 2016
- views: 1
- published: 12 Mar 2016
- views: 1
Hello - song by Lionel Richie played with atomic nuclei
- Order: Reorder
- Duration: 4:34
- Updated: 12 Mar 2016
- views: 1
- published: 12 Mar 2016
- views: 1
FROM ATOMIC NUCLEI TO STARS!
- Order: Reorder
- Duration: 90:47
- Updated: 28 Jul 2014
- views: 37
- published: 28 Jul 2014
- views: 37
Half Moon Bay - song by Dan Fogelberg played with atomic nuclei
- Order: Reorder
- Duration: 1:38
- Updated: 13 Mar 2016
- views: 0
- published: 13 Mar 2016
- views: 0
Dr Shivago - movie title song played by atomic nuclei
- Order: Reorder
- Duration: 0:50
- Updated: 12 Mar 2016
- views: 0
- published: 12 Mar 2016
- views: 0
Formation of the Atomic Nuclei
- Order: Reorder
- Duration: 5:06
- Updated: 30 Apr 2015
- views: 263
- published: 30 Apr 2015
- views: 263
Photoelectric Effect | Modern Physics (Atoms, Nuclei)
- Order: Reorder
- Duration: 7:28
- Updated: 07 May 2015
- views: 668
- published: 07 May 2015
- views: 668
Forces in atomic nuclei (with subtitles)
- Order: Reorder
- Duration: 9:00
- Updated: 20 Mar 2010
- views: 687
- published: 20 Mar 2010
- views: 687
Teach Astronomy - Energy from Atomic Nuclei
- Order: Reorder
- Duration: 0:59
- Updated: 09 Jul 2010
- views: 124
- published: 09 Jul 2010
- views: 124
Chemistry: The Stability of Atomic Nuclei
- Order: Reorder
- Duration: 4:03
- Updated: 17 Feb 2011
- views: 397
- published: 17 Feb 2011
- views: 397
-

Demos Lord. "Nuclei atomic dissolutionem". de verbum lucet
Publicado em 11 de mar de 2016 A palidez intensa da face, que observando a cada fusão de átomos, escoria um suor frio. A perda mais ou menos -+ grave da força muscular, e o relaxamento dos nervos, "e descobriu através dos raios catódicos... Tudo que atinge do solo ao Céu, a atmosfera reduzida seus eletros, a saúde do corpo instável os pensamentos em distúrbio até o completo abandono do corpo e queda:. Com a descoberta do nêutron ficou claro que deveriam existir muitas possibilidades dessas modificações. (https://www.youtube.com/editor) (https://www.youtube.com/editor) -

Demos Lordis "Nuclei atomic dissolutionem".
Tudo que atinge do solo ao Céu, a atmosfera redusida seus eletros, a saúde do corpo instavel os pensamentos em distúrbio até o completo abandono do corpo e queda:. Com a descoberta do nêutron ficou claro que deveriam existir muitas possibilidades dessas modificações. (https://www.youtube.com/editor) -

"Understanding the Atomic Nucleus" by Ruprecht Machleidt
00:08 Ruprecht Machleidt (Department of Physics, University of Idaho (USA)) 53:48 Public interventions "Understanding the Atomic Nucleus: Recent Dramatic Advances and Remaining Challenges" ---------- Abstract: ---------- For about 80 years, physicists have worked on understanding the atomic nucleus in fundamental terms, but only the past decade has brought about a dramatic breakthrough towards achieving this goal. The fundamental (“microscopic”, “ab initio”) approach has two ingredients: the basic forces between nucleons and quantum many-body theory. Since the nuclear force is a manifestation of strong interactions, any serious derivation has to start from quantum chromodynamics (QCD). However, QCD is non-perturbative at low energies, which renders its application to nuclear physics ... -

A Level Physics - The Size, Mass and Density of the Nucleus
Find out a little more about the masses of the subatomic particles and the Atomic Mass Unit, u. You can also estimate the size of the atomic nuclei and therefore calculate their density which is massive! If you would like to see more A Level Physics videos then please Subscribe to my channel to keep updated with new videos and to search the Playlists already created. You can also visit my site 'A Level Physics Online' to see how all the videos relate to your course and for even more resources at http://www.alevelphysicsonline.com/ Thanks for watching, Mr Matheson -

Nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei.It determines the physical and chemical properties of atoms or the molecules in which they are contained. =======Image-Copyright-Info======= Image is in public domain Author-Info: MartinSaunders at English Wikipedia Image Source: https://en.wikipedia.org/wiki/File:HWB-NMR_-_900MHz_-_21.2_Tesla.jpg =======Image-Copyright-Info======== -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=2xuXuDI0Fkc -

Even and odd atomic nuclei
Even and odd atomic nuclei In nuclear physics, properties of a nucleus depend on evenness or oddness of its atomic number Z, neutron number N and, consequently, of their sum, the mass number A.Most notably, oddness of both Z and N tends to lower the nuclear binding energy, making odd nuclei, generally, less stable. =======Image-Copyright-Info======== License: Creative Commons Attribution-Share Alike 3.0 (CC BY-SA 3.0) LicenseLink: http://creativecommons.org/licenses/by-sa/3.0 Author-Info: Michalsmid Image Source: https://en.wikipedia.org/wiki/File:NuclearReaction.png =======Image-Copyright-Info======== -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=r9ihEZJBOis -

Nuclear engineering
Nuclear engineering is the branch of engineering concerned with the application of the breakdown as well as the fusion of atomic nuclei and/or the application of other sub-atomic physics, based on the principles of nuclear physics. In the sub-field of nuclear fission, it particularly includes the interaction and maintenance of systems and components like nuclear reactors, nuclear power plants, and/or nuclear weapons. The field also includes the study of medical and other applications of radiation, nuclear safety, heat/thermodynamics transport, nuclear fuel and/or other related technology, and the problems of nuclear proliferation. This video is targeted to blind users. Attribution: Article text available under CC-BY-SA Creative Commons image source in video -

Class 12, Physics,Lec7-Orbital Velocity(Atom & Nuclei) XII
Concept of orbital velocity of an electron with the help of Bohr’s concept of hydrogen model Class 12, Physics,Lec7-Orbital Velocity(Atom & Nuclei) CBSE Class XI,Class XII,Physics,Chemistry,Biology NCERT Physics,Chemistry,Biology Buy Full Video For Rs. 200 At www.sci4you.com -

Physics | Atoms, Molecules and Nuclei | CET (15th December 2015)
Atoms, Molecules and Nuclei | CET Centre : ANDHERI,BORIVALI,DADAR,NERUL,THANE,KHARGHAR,CHEMBUR,POWAI Date : 15th December 2015 Faculty : HNP Subscribe Now - https://www.youtube.com/channel/UCFvb... Watch this Lecturer and more in my 'Daily VC Lecture' playlist: https://www.youtube.com/playlist?list... Visit my channel for more videos: https://www.youtube.com/paceiit&m...; Social Media links: FaceBook https://www.facebook.com/PaceEducare/... Twitter https://twitter.com/paceeducare PACE IIT & MEDICAL http://www.iitianspace.com/index.php -

SC200 Unit 4 Quiz (Kaplan)
BUY HERE⬊ http://www.homeworkmade.com/kaplan-10/sc200/sc200-unit-4-quiz-kaplan/ 1. The Yucca Mountain project for nuclear waste disposal 2. What kind of energy does the pounding of ocean surf demonstrate? 3. James Watt designed experiments in horsepower to: 4. The combination of two atomic nuclei is referred to as fusion, whereas the splitting of a single atomic nucleus is called 5. Thermal energy is measured in units of 6. Einstein's equation, E = mc2 , states that mass can be converted into 7. A particular isotope has a half-life of 10 minutes. If we start with 2,000 atoms now, in a half hour we will have how many atoms left? 8. Which particle found in the nucleus of the atom has no electrical charge? 9. Energy can be stored in a flashlight battery as: 10. When a meteorite hits Earth, he... -

Atoms , molecules and nuclei HSC Physics Maharashtra Board,MHT CET std 12 , Lect 1
Pune's Leading Coaching Class For MHT-CET ,Engineering And Medical Entrances. Maharashtra Board -

Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons, primarily protons and neutrons. The first nuclei were formed about three minutes after the Big Bang, through the process called Big Bang nucleosynthesis. It was then that hydrogen and helium formed to become the content of the first stars, and this primeval process is responsible for the present hydrogen/helium ratio of the cosmos. With the formation of stars, heavier nuclei were created from hydrogen and helium by stellar nucleosynthesis, a process that continues today. Some of these elements, particularly those lighter than iron, continue to be delivered to the interstellar medium when low mass stars eject their outer envelope before they collapse to form white dwarfs. The remains of their ejected m... -

Nuclear force
The nuclear force is the force between protons and neutrons, subatomic particles that are collectively called nucleons. The nuclear force is responsible for binding protons and neutrons into atomic nuclei. Neutrons and protons are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience a strong electric field repulsion that tends to push them apart, but at short range the attractive nuclear force overcomes the repulsive electromagnetic force. The mass of a nucleus is less than the sum total of the individual masses of the protons and neutrons which form it. The difference in mass between bound and unbound nucleons is known as the mass defect. Energy is released when some large nuclei break apart, and it is this energy that is used in nuclear power ... -

Fermi Telescope Proves Supernova Remnants Produce Cosmic Rays
A study using observations from NASA's Fermi Gamma-ray Space Telescope reveals the first clear-cut evidence that the expanding debris of exploded stars produces some of the fastest-moving matter in the universe. This discovery is a major step toward meeting one of Fermi's primary mission goals. Cosmic rays are subatomic particles that move through space at nearly the speed of light. About 90 percent of them are protons, with the remainder consisting of electrons and atomic nuclei. In their journey across the galaxy, the electrically charged particles become deflected by magnetic fields. This scrambles their paths and makes it impossible to trace their origins directly. This video is public domain and can be downloaded at: http://svs.gsfc.nasa.gov/goto?11209 credit: NASA's Goddard Space... -

CERN Collides Heavy Nuclei at New Record High Energy
www.undergroundworldnews.com The world's most powerful accelerator, the 27 km long Large Hadron Collider (LHC) operating at CERN in Geneva established collisions between lead nuclei, this morning, at the highest energies ever. The LHC has been colliding protons at record high energy since the summer, but now the time has now come to collide large nuclei (nuclei of lead, Pb, consist of 208 neutrons and protons). The experiments aim at understanding and studying the properties of strongly interacting systems at high densities and thus the state of matter of the Universe shortly after the Big Bang. In the very beginning, just a few billionths of a second after the Big Bang, the Universe was made up of an extremely hot and dense 'primordial soup' consisting of the fundamental particles, espe... -

Protons and Neutrons: Quarks 3D
A quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. -

nuclear physics - Dictiome pronunciation database
How to pronounce "nuclear physics" in English? "nuclear physics" usage and pronunciation. Watch more at http://www.dictiome.com Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions. The most commonly known application of nuclear physics is nuclear power generation, but the research has led to applications in many fields, including nuclear medicine and magnetic resonance imaging, nuclear weapons, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. The field of particle physics evolved out of nuclear physics and is typically tau... http://www.dictiome.com/en/63931/nuclear-physics-pronunciation-Aussprache-prononciation-%D0%BF%D1%80%D0%BE%D0%B8%D0%B7%D0%BD%D0%BE%D1%88%D0%B5%D0%BD%D0%B8%D0%B5-pronu... -

Electromagnetic Response of Atomic Nuclei Oxford Studies in Nuclear Physics PDF
http://www.realbooknow.net/books
Demos Lord. "Nuclei atomic dissolutionem". de verbum lucet
- Order: Reorder
- Duration: 11:17
- Updated: 14 Mar 2016
- views: 4
- published: 14 Mar 2016
- views: 4
Demos Lordis "Nuclei atomic dissolutionem".
- Order: Reorder
- Duration: 2:42
- Updated: 11 Mar 2016
- views: 4
- published: 11 Mar 2016
- views: 4
"Understanding the Atomic Nucleus" by Ruprecht Machleidt
- Order: Reorder
- Duration: 59:03
- Updated: 10 Feb 2016
- views: 35
- published: 10 Feb 2016
- views: 35
A Level Physics - The Size, Mass and Density of the Nucleus
- Order: Reorder
- Duration: 4:18
- Updated: 02 Feb 2016
- views: 88
- published: 02 Feb 2016
- views: 88
Nuclear magnetic resonance spectroscopy
- Order: Reorder
- Duration: 21:21
- Updated: 29 Jan 2016
- views: 0
- published: 29 Jan 2016
- views: 0
Even and odd atomic nuclei
- Order: Reorder
- Duration: 12:17
- Updated: 22 Jan 2016
- views: 4
- published: 22 Jan 2016
- views: 4
Nuclear engineering
- Order: Reorder
- Duration: 4:52
- Updated: 14 Jan 2016
- views: 7
- published: 14 Jan 2016
- views: 7
Class 12, Physics,Lec7-Orbital Velocity(Atom & Nuclei) XII
- Order: Reorder
- Duration: 5:02
- Updated: 10 Jan 2016
- views: 40
- published: 10 Jan 2016
- views: 40
Physics | Atoms, Molecules and Nuclei | CET (15th December 2015)
- Order: Reorder
- Duration: 85:23
- Updated: 22 Dec 2015
- views: 174
- published: 22 Dec 2015
- views: 174
SC200 Unit 4 Quiz (Kaplan)
- Order: Reorder
- Duration: 0:08
- Updated: 14 Dec 2015
- views: 5
- published: 14 Dec 2015
- views: 5
Atoms , molecules and nuclei HSC Physics Maharashtra Board,MHT CET std 12 , Lect 1
- Order: Reorder
- Duration: 19:55
- Updated: 14 Dec 2015
- views: 222
- published: 14 Dec 2015
- views: 222
Nucleosynthesis
- Order: Reorder
- Duration: 25:40
- Updated: 14 Dec 2015
- views: 12
- published: 14 Dec 2015
- views: 12
Nuclear force
- Order: Reorder
- Duration: 18:21
- Updated: 14 Dec 2015
- views: 17
- published: 14 Dec 2015
- views: 17
Fermi Telescope Proves Supernova Remnants Produce Cosmic Rays
- Order: Reorder
- Duration: 3:40
- Updated: 08 Dec 2015
- views: 41
- published: 08 Dec 2015
- views: 41
CERN Collides Heavy Nuclei at New Record High Energy
- Order: Reorder
- Duration: 2:37
- Updated: 06 Dec 2015
- views: 28647
- published: 06 Dec 2015
- views: 28647
Protons and Neutrons: Quarks 3D
- Order: Reorder
- Duration: 0:16
- Updated: 04 Dec 2015
- views: 29
- published: 04 Dec 2015
- views: 29
nuclear physics - Dictiome pronunciation database
- Order: Reorder
- Duration: 0:41
- Updated: 03 Dec 2015
- views: 4
- published: 03 Dec 2015
- views: 4
Electromagnetic Response of Atomic Nuclei Oxford Studies in Nuclear Physics PDF
- Order: Reorder
- Duration: 0:26
- Updated: 30 Nov 2015
- views: 2
-

Class 12 Physics - Nucleus
In class 12th CBSE Physics syllabus one of the topic is 'Nuclei'. In this chapter we will study about Composition and size of nucleus, atomic masses, isotopes, isobars ,isotones. Apart from it ,this chapter includes Radioactivity alpha, beta and gamma particles/rays and their properties, radioactive decay law, Mass-energy relation, mass defect, binding energy per nucleon and its variation with mass number, nuclear fission and nuclear fusion. Here is a demo of online video lecture. You can watch this complete video at http://www.dronstudy.com Alternatively, visit this URL to access this chapter directly http://dronstudy.com/video/class-xii-nuclei ..... Here is the list of important URLs related to dronstudy.com Youtube Video Link: http://www.youtube.com/edit?o=U&video;_id=ixXHP3dpado ... -

-

-

Atomic Physics: 16. Nuclei and Particles: 12: Particle Physics & Standard Model
Last part of a video lecture on atomic nuclei and particles, sketching briefly some basic ideas about elementary particles, their interactions, and the standard model of particle physics. This part concludes the lecture and the entire lecture series on atomic and quantum physics and the structure of matter. Recorded TU Darmstadt, Germany, during the summer of 2010. Language: English Free video in HD quality available at http://openlearnware.tu-darmstadt.de -

-

Nucleus - IIT JEE Main and Advanced Physics Video Lecture [RAO IIT ACADEMY]
IIT JEE Main and Advanced Physics Video Lectures and Study Material developed by highly experienced and dedicated faculty team of Rao IIT Academy. Visit http://www.raoiit.com or email studentcare@raoiit.com for any query or doubt related to your IIT JEE Preparation. Want to be the Topper ??? Learn Nucleus from Rao IIT Academy. Prepare for your Boards and New IIT-JEE Pattern - JEE Main and JEE Advanced / MH-CET / BITSAT / VIT / SRM / Manipal and other Competitive Exams with Rao IIT Academy. ...................................... Subscribe to Rao IIT Academy YouTube channel - http://www.youtube.com/RaoIITAcademy Like us on Facebook - https://www.facebook.com/Raoiit Follow us on Twitter - https://twitter.com/rao_iit +1 on Google Plus - https://plus.google.com/+Raoiit Call us on: +91-9699-32... -

Hangout with CERN: Going pear-shaped (S03E05)
In this week's hangout we're joined by scientists from the ISOLDE facility at CERN to find out their latest news of pear-shaped atomic nuclei and a fundamental property of the rarest element on Earth. Host ATLAS physicist Steven Goldfarb is joined by Bruce Marsh and Valentine Fedosseev, in the ISOLDE visitor room are Liam Gaffney, KU Leuven, Thomas Cocolios, University of Manchester and Fredrik Wenander, CERN; Mark Huyse connecting from KU Leuven, with Achintya Rao from the CMS experiment monitoring social media. Read more about these latest results here: http://home.web.cern.ch/about/updates/2013/05/first-observations-short-lived-pear-shaped-atomic-nuclei and http://home.web.cern.ch/about/updates/2013/05/fundamental-property-rarest-element-earth Recorded live on 23rd May 2013. The han... -

-

PY106 pre-class video for session 42 - The nucleus
In this video, we talk about some general features of the atomic nucleus, such as what it is made up of, how large it is, and what keeps it together. We will also look at the idea of the mass defect, which is related to the binding energy of the nucleus. The video includes a calculation of the mass defect of a carbon-12 atom. -

How to Make an Atomic Bomb | Doc Physics
Firstly, don't call it atomic. It should be called a nuclear bomb, 'cuz you're messing with nuclei. Secondly, don't make a bomb. Instead, learn about fission and fusion so you can solve humanity's energy problems and make life better for others. -

R. F. Casten @CERN - Lecture 1
A series of four nuclear physics lectures given by Prof. Richard Casten from Yale University. These lectures will provide a simple overview of the structure of atomic nuclei, including empirical signatures of structure, the dependence of structure on proton and neutron numbers, the complementarity of microscopic ( nucleon-based) and macroscopic (many-body and symmetry perspective) approaches. After an overview of nuclear structure and a discussion of the shell model and standard collective models in the first two lectures, the microscopic drivers of collectivity, nuclear shape evolution and phase transitions will be discussed in the 3rd lecture.The 4th lecture will focus on the implications of these ideas for exotic nuclei and the opportunities they present. Started 23 Jul 2013 14:00 Ended... -

Physics@FOM Veldhoven 2016 - Masterclass Laura Baudis
Prior to Physics@FOM Veldhoven 2016, the programme committee organised four masterclasses. These classes offer PhDs and young postdocs a unique opportunity to receive an introduction to their discipline from top researchers. The state-of-the-art in the search for dark matter One of the major challenges of modern physics is to decipher the nature of dark matter. Astrophysical observations provide ample evidence for the existence of an invisible and dominant mass component in the observable universe, from the scales of galaxies up to the largest cosmological scales. The dark matter could be made of new, yet undiscovered elementary particles, with allowed masses and interaction strengths with normal matter spanning an enormous range. Weakly interacting massive particles (WIMPs), which fro... -

Nuclear fusion and the future of energy
Speakers: Bob Bingham; Joe Kaplinsky; Michael Massey; Alexandra Penn Chair: Rob Clowes For several decades now, the possibility of nuclear fusion -- producing energy by joining atomic nuclei rather than splitting them -- has held the promise of cheap and clean power for all, without the waste produced by existing fission technology. But somehow fusion is rarely high on the agenda in debates about the future of energy. Arguably, recent technical innovations indicate a working fusion reactor is now a realisable project in the medium term. Several major projects - such as the European HiPER project -- now exist with the goal of advancing this dream over the next ten years. Yet, perhaps surprisingly, the promise of cheap clean energy has not been universally welcomed. Some argue we have be... -

Frank Wilczek
Frank Wilczek Herman Feshbach (1942) Professor of Physics Nobel laureate in Physics, 2004 MacArthur Fellow Frank Wilczek, considered one of the world's most eminent theoretical physicists, is the Herman Feshbach professor of physics at MIT. He received his Bachelor’s degree from the University of Chicago and his PhD from Princeton University. When only 21 years old and a graduate student at Princeton working with David Gross, Professor Wilczek defined the properties of color gluons, which hold atomic nuclei together. He has received numerous awards for his work, including the 2004 Nobel Prize in Physics for the discovery of asymptotic freedom in the theory of the strong interaction. He is also a distinguished science writer and poet. -

The Speed of Light: Cumulative Instantaneous Forces at a Distance
Ralph Sansbury - Light itself need not be produced by instantaneous transitions between energy levels and then propagated as a wave or photon or a probabilistic photon with a velocity equal to the speed of light. Instead, light or radiation in general, may be regarded as the effect of oscillations of charged particles in a source that produce at a distance in-phase oscillations of charged particles in the primary receiver, first inside atomic nuclei, then after a delay, oscillations of electrons, e.g., of free electrons or of bound electrons where the widening of orbits of bound atomic electrons leads to their ejection. The proposed mechanism to produce such light transmission is similar to Maxwell\'s changing electric fields causing magnetic fields and changing magnetic fields causing ele... -

Physics@FOM Veldhoven 2016, Laura Baudis - The state-of-the-art in the search for dark matter
The state-of-the-art in the search for dark matter One of the major challenges of modern physics is to decipher the nature of dark matter. Astrophysical observations provide ample evidence for the existence of an invisible and dominant mass component in the observable universe, from the scales of galaxies up to the largest cosmological scales. The dark matter could be made of new, yet undiscovered elementary particles, with allowed masses and interaction strengths with normal matter spanning an enormous range. Weakly interacting massive particles (WIMPs), which froze out of thermal equilibrium with a relic density matching the observations, represent a well-motivated, generic classes of dark matter candidates. They could be directly observed via scatters off atomic nuclei in underground, u... -

-

Patterns of decay
How does a random decay give rise to predictable properties? When a large number of nuclei are involved, statistics can be used to make predictions about the rate of decay of atomic nuclei. In this video, I derive the decay equation from first principles and look at half life and the decay constant. This video is produced to cover the content of the OCR A specification of A level Physics. -

√ Decay and Transmutations - Nuclear Decay - From Quanta to Quarks - Nuclear Physics | iitutor
https://www.iitutor.com Transmutation is the process responsible for transforming one element into another. There are two fundamentally different processes whereby transmutations occur: nuclear decay and nuclear fusion. Nuclear decay occurs when there is the emission of an alpha or beta particle from the nucleus. These are known as alpha and beta decay respectively when they occur naturally. Natural transmutations involve the emission of alpha or beta particles from the nucleus of a radioactive atom. In the early days of studying radioactive decay, one of the first devices invented was the Wilson cloud chamber by Scottish scientist Charles Wilson. The penetration of the alpha or beta particle through a saturated vapour in a sealed chamber results in a cloud-like track of condensation. If t...
Class 12 Physics - Nucleus
- Order: Reorder
- Duration: 29:56
- Updated: 15 Jan 2014
- views: 2593
- published: 15 Jan 2014
- views: 2593
XII-12-1 Atom part-1(2015)
- Order: Reorder
- Duration: 80:11
- Updated: 17 Nov 2015
- views: 2568
XII-12-2 Atom part-2 (2015)
- Order: Reorder
- Duration: 78:00
- Updated: 16 Nov 2015
- views: 3607
Atomic Physics: 16. Nuclei and Particles: 12: Particle Physics & Standard Model
- Order: Reorder
- Duration: 20:57
- Updated: 29 Jan 2012
- views: 1726
- published: 29 Jan 2012
- views: 1726
ATOM AND NUCLEI PART 1
- Order: Reorder
- Duration: 56:08
- Updated: 09 Oct 2013
- views: 256
Nucleus - IIT JEE Main and Advanced Physics Video Lecture [RAO IIT ACADEMY]
- Order: Reorder
- Duration: 29:15
- Updated: 15 May 2014
- views: 2205
- published: 15 May 2014
- views: 2205
Hangout with CERN: Going pear-shaped (S03E05)
- Order: Reorder
- Duration: 31:17
- Updated: 23 May 2013
- views: 9275
- published: 23 May 2013
- views: 9275
XII-10.3.Atom,Bohrs Postulates Pradeep Kshetrapal Physics
- Order: Reorder
- Duration: 74:43
- Updated: 25 Nov 2014
- views: 9107
PY106 pre-class video for session 42 - The nucleus
- Order: Reorder
- Duration: 22:34
- Updated: 26 Apr 2011
- views: 1847
- published: 26 Apr 2011
- views: 1847
How to Make an Atomic Bomb | Doc Physics
- Order: Reorder
- Duration: 20:07
- Updated: 22 Apr 2013
- views: 18341
- published: 22 Apr 2013
- views: 18341
R. F. Casten @CERN - Lecture 1
- Order: Reorder
- Duration: 36:54
- Updated: 29 Jul 2013
- views: 466
- published: 29 Jul 2013
- views: 466
Physics@FOM Veldhoven 2016 - Masterclass Laura Baudis
- Order: Reorder
- Duration: 129:55
- Updated: 22 Feb 2016
- views: 15
- published: 22 Feb 2016
- views: 15
Nuclear fusion and the future of energy
- Order: Reorder
- Duration: 65:12
- Updated: 27 Jul 2010
- views: 1200
- published: 27 Jul 2010
- views: 1200
Frank Wilczek
- Order: Reorder
- Duration: 108:42
- Updated: 08 Mar 2016
- views: 29
- published: 08 Mar 2016
- views: 29
The Speed of Light: Cumulative Instantaneous Forces at a Distance
- Order: Reorder
- Duration: 32:45
- Updated: 08 Aug 2012
- views: 1293
- published: 08 Aug 2012
- views: 1293
Physics@FOM Veldhoven 2016, Laura Baudis - The state-of-the-art in the search for dark matter
- Order: Reorder
- Duration: 39:09
- Updated: 12 Feb 2016
- views: 10
- published: 12 Feb 2016
- views: 10
XII_92. Nuclei, Radioactivity
- Order: Reorder
- Duration: 46:27
- Updated: 03 Nov 2012
- views: 16141
Patterns of decay
- Order: Reorder
- Duration: 24:57
- Updated: 23 Mar 2014
- views: 205
- published: 23 Mar 2014
- views: 205
√ Decay and Transmutations - Nuclear Decay - From Quanta to Quarks - Nuclear Physics | iitutor
- Order: Reorder
- Duration: 21:09
- Updated: 07 Mar 2014
- views: 2098
- published: 07 Mar 2014
- views: 2098
- Playlist
- Chat
- Playlist
- Chat

Physical Science 7.4c - The Atomic Nucleus
- Report rights infringement
- published: 27 Jul 2009
- views: 46793

Atomic Nucleus
- Report rights infringement
- published: 04 May 2014
- views: 22330

Atomic Nucleus
- Report rights infringement
- published: 10 Sep 2010
- views: 10019

The Discovery of the Atomic Nucleus (3 of 15)
- Report rights infringement
- published: 01 Mar 2008
- views: 273684

The Nucleus: Crash Course Chemistry #1
- Report rights infringement
- published: 12 Feb 2013
- views: 1990036

Baths and Quarks: Solitons explained
- Report rights infringement
- published: 17 Feb 2012
- views: 39287

39 1 The Atomic Nucleus and Radioactivity
- Report rights infringement
- published: 20 Mar 2014
- views: 1576

Neutron stars - A melting pot for atomic nuclei
- Report rights infringement
- published: 10 Jan 2014
- views: 2306


Collisions of Elementary Particles with Atomic Nuclei - Roy Glauber
- Report rights infringement
- published: 03 Sep 2014
- views: 244

Just How Small is an Atom?
- Report rights infringement
- published: 16 Apr 2012
- views: 1768950

Ferry Cross the Mersey - played with atomic nuclei
- Report rights infringement
- published: 13 Mar 2016
- views: 4

Designer Atomic Nuclei: Interview with NSCL's Brad Sherrill
- Report rights infringement
- published: 09 May 2008
- views: 633

Against All Odds - sounds from atomic nuclei
- Report rights infringement
- published: 12 Mar 2016
- views: 1
- Playlist
- Chat

Demos Lord. "Nuclei atomic dissolutionem". de verbum lucet
- Report rights infringement
- published: 14 Mar 2016
- views: 4

Demos Lordis "Nuclei atomic dissolutionem".
- Report rights infringement
- published: 11 Mar 2016
- views: 4

"Understanding the Atomic Nucleus" by Ruprecht Machleidt
- Report rights infringement
- published: 10 Feb 2016
- views: 35

A Level Physics - The Size, Mass and Density of the Nucleus
- Report rights infringement
- published: 02 Feb 2016
- views: 88

Nuclear magnetic resonance spectroscopy
- Report rights infringement
- published: 29 Jan 2016
- views: 0

Even and odd atomic nuclei
- Report rights infringement
- published: 22 Jan 2016
- views: 4

Nuclear engineering
- Report rights infringement
- published: 14 Jan 2016
- views: 7

Class 12, Physics,Lec7-Orbital Velocity(Atom & Nuclei) XII
- Report rights infringement
- published: 10 Jan 2016
- views: 40

Physics | Atoms, Molecules and Nuclei | CET (15th December 2015)
- Report rights infringement
- published: 22 Dec 2015
- views: 174

SC200 Unit 4 Quiz (Kaplan)
- Report rights infringement
- published: 14 Dec 2015
- views: 5

Atoms , molecules and nuclei HSC Physics Maharashtra Board,MHT CET std 12 , Lect 1
- Report rights infringement
- published: 14 Dec 2015
- views: 222

Nucleosynthesis
- Report rights infringement
- published: 14 Dec 2015
- views: 12

Nuclear force
- Report rights infringement
- published: 14 Dec 2015
- views: 17

Fermi Telescope Proves Supernova Remnants Produce Cosmic Rays
- Report rights infringement
- published: 08 Dec 2015
- views: 41
- Playlist
- Chat

Class 12 Physics - Nucleus
- Report rights infringement
- published: 15 Jan 2014
- views: 2593



Atomic Physics: 16. Nuclei and Particles: 12: Particle Physics & Standard Model
- Report rights infringement
- published: 29 Jan 2012
- views: 1726

ATOM AND NUCLEI PART 1
- Report rights infringement
- published: 09 Oct 2013
- views: 256

Nucleus - IIT JEE Main and Advanced Physics Video Lecture [RAO IIT ACADEMY]
- Report rights infringement
- published: 15 May 2014
- views: 2205

Hangout with CERN: Going pear-shaped (S03E05)
- Report rights infringement
- published: 23 May 2013
- views: 9275


PY106 pre-class video for session 42 - The nucleus
- Report rights infringement
- published: 26 Apr 2011
- views: 1847

How to Make an Atomic Bomb | Doc Physics
- Report rights infringement
- published: 22 Apr 2013
- views: 18341

R. F. Casten @CERN - Lecture 1
- Report rights infringement
- published: 29 Jul 2013
- views: 466

Physics@FOM Veldhoven 2016 - Masterclass Laura Baudis
- Report rights infringement
- published: 22 Feb 2016
- views: 15

Nuclear fusion and the future of energy
- Report rights infringement
- published: 27 Jul 2010
- views: 1200

Frank Wilczek
- Report rights infringement
- published: 08 Mar 2016
- views: 29
Trump presidency rated among top 10 global risks: EIU
Edit BBC News 17 Mar 2016Scientists Solve 58-Year-Old Mystery Surrounding Alien-Looking Tully Monster
Edit WorldNews.com 16 Mar 2016Discovery Of Oldest Human DNA Could Rewrite Evolutionary History Of Mankind
Edit WorldNews.com 16 Mar 2016'Holy grail' set of Shakespeare folios for sale
Edit Jakarta Post 17 Mar 2016While the West was applying pressure on Iran to abandon its civilian nuclear programme, the Saudis were buying the atomic bomb from Israel or Pakistan. By Thierry Meyssan
Edit Information Clearing House 17 Mar 2016Scientists Discover Pregnant T. Rex Fossil
Edit IFL Science 17 Mar 2016Most powerful source of cosmic radiation (University of the Witwatersrand)
Edit Public Technologies 17 Mar 2016Notre Dame Academy junior to represent US at youth conference in Japan
Edit Topix 17 Mar 2016Officer on leave over controversial Facebook post
Edit San Francisco Chronicle 17 Mar 2016Prakash Karat
Edit NDTV 17 Mar 2016Buhari Commends IAEA's Support for Nigeria's Nuclear Electricity Generation
Edit All Africa 17 Mar 2016Power - Buhari Welcomes Int'l Energy Agency's Aid
Edit All Africa 17 Mar 2016Cop sorry for image of mushroom cloud, reference to Islam
Edit Denver Post 17 Mar 2016China makes breakthrough in 'man-made sun' research
Edit China Daily 17 Mar 2016Capturing ‘black gold’ with light (Monash University)
Edit Public Technologies 17 Mar 2016- 1
- 2
- 3
- 4
- 5
- Next page »







