- published: 28 Sep 2013
- views: 34836
-
remove the playlistIon Exchange
- remove the playlistIon Exchange
- published: 13 Sep 2012
- views: 177766
- published: 09 Apr 2013
- views: 62653
- published: 03 Feb 2015
- views: 25027
- published: 29 Sep 2013
- views: 20877
- published: 27 Oct 2012
- views: 42692
- published: 17 May 2014
- views: 44679
- published: 18 Jan 2016
- views: 307

Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion exchangers'.
Typical ion exchangers are ion exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion exchangers are either cation exchangers that exchange positively charged ions (cations) or anion exchangers that exchange negatively charged ions (anions). There are also amphoteric exchangers that are able to exchange both cations and anions simultaneously. However, the simultaneous exchange of cations and anions can be more efficiently performed in mixed beds that contain a mixture of anion and cation exchange resins, or passing the treated solution through several different ion exchange materials.
Ion exchangers can be unselective or have binding preferences for certain ions or classes of ions, depending on their chemical structure. This can be dependent on the size of the ions, their charge, or their structure. Typical examples of ions that can bind to ion exchangers are:
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 9:49
9:49IonExchange
IonExchangeIonExchange
How ion exchange can be used to soften hard water. -
 1:41
1:41The Principle of Ion Exchange Chromatography
The Principle of Ion Exchange ChromatographyThe Principle of Ion Exchange Chromatography
Download our free 187-page handbook "Ion Exchange Chromatography" http://www.gelifesciences.com/iexhandbook All our handbooks are available here: http://www.gelifesciences.com/handbooks Ion exchange chromatography is one of the most frequently used techniques for purification of biomolecules and separates the molecules according to differences in their net surface charge. This video describes the principles of the technique. -
 4:51
4:51How an ion exchange water softener works - San Antonio TX
How an ion exchange water softener works - San Antonio TXHow an ion exchange water softener works - San Antonio TX
http://texaswatersoftener.com So you know the benefits of a San Antonio water softening system, but you aren't sure how it works. This video explains the ion exchange process along with an animation. Please visit our web site for more information on water softeners and browse our reviews and comparisons. -
 10:29
10:29Ion Exchange Chromatography
Ion Exchange ChromatographyIon Exchange Chromatography
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/ion-exchange-chromatography Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com -
 6:22
6:22Ion exchange chromatography
Ion exchange chromatographyIon exchange chromatography
-
 1:25
1:25Ion Exchange Chromatography
Ion Exchange ChromatographyIon Exchange Chromatography
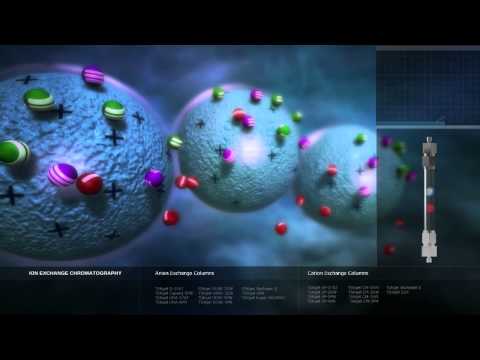
Illustration of Ion Exchange Chromatography and how it works on a particle level. IEC is a very powerful and selective separation mode. Biomolecules generally have charged groups on their surfaces. The overall charge changes with the pH of the solution. This is the basis for Ion Exchange Chromatography, in which molecules bind to a charged group of the stationary phase. More information can be found here http://www.separations.eu.tosohbioscience.com/ServiceSupport/TechSupport/ResourceCenter/PrinciplesofChromatography/IonExchange -
 8:45
8:45Ion exchange chromatography
Ion exchange chromatographyIon exchange chromatography
This chromatography lecture explains about ion exchange chromatography.Web- http://shomusbiology.weebly.com/ Download the study materials here- http://shomusbiology.weebly.com/bio-materials.html This video tutorial explains the mechanisms of ion exchange chromatography and its use in biochemistry -
 4:23
4:23Ion Exchange Chromatography
Ion Exchange Chromatography -
 6:46
6:46Ion Exchange Chromatography
Ion Exchange ChromatographyIon Exchange Chromatography
-
 3:37
3:37022-Ion Exchange Chromatography
022-Ion Exchange Chromatography022-Ion Exchange Chromatography
Discussion of principles behind ion exchange chromatography -
 6:58
6:58Ion Exchange Chromatography - Theory and Principle
Ion Exchange Chromatography - Theory and PrincipleIon Exchange Chromatography - Theory and Principle
http://technologyinscience.blogspot.com/2013/07/an-insight-on-ion-exchange.html The principle of Ion Exchange chromatography separation is the reversible interaction of charged species with the ion exchange matrix. Ion Exchange Chromatography is of Two types: Cation Exchange and Anion Exchange. -
 14:59
14:59Ion exchange chromatography | cation exchange chromatography and anion exchange chromatography
Ion exchange chromatography | cation exchange chromatography and anion exchange chromatographyIon exchange chromatography | cation exchange chromatography and anion exchange chromatography
This comment about the video lecture explains about ion exchange chromatography principle. It also explains the step-by-step process of ion exchange chromatography. That includes the the cation exchange chromatography and anion exchange chromatography. Ion exchange chromatography involves using positive and negative in charge ions to separate charged molecules from a mixture. In the positive this is a problem was in video. For more information, log on to- http://www.shomusbiology.com/ Get Shomu's Biology DVD set here- http://www.shomusbiology.com/dvd-store/ Download the study materials here- http://shomusbiology.com/bio-materials.html Remember Shomu’s Biology is created to spread the knowledge of life science and biology by sharing all this free biology lectures video and animation presented by Suman Bhattacharjee in YouTube. All these tutorials are brought to you for free. Please subscribe to our channel so that we can grow together. You can check for any of the following services from Shomu’s Biology- Buy Shomu’s Biology lecture DVD set- www.shomusbiology.com/dvd-store Shomu’s Biology assignment services – www.shomusbiology.com/assignment -help Join Online coaching for CSIR NET exam – www.shomusbiology.com/net-coaching We are social. Find us on different sites here- Our Website – www.shomusbiology.com Facebook page- https://www.facebook.com/ShomusBiology/ Twitter - https://twitter.com/shomusbiology SlideShare- www.slideshare.net/shomusbiology Google plus- https://plus.google.com/113648584982732129198 LinkedIn - https://www.linkedin.com/in/suman-bhattacharjee-2a051661 Youtube- https://www.youtube.com/user/TheFunsuman Thank you for watching -
 4:25
4:25Ion Exchange Column
Ion Exchange ColumnIon Exchange Column
This video demonstrates the use of a cation exchange column to convert a salt to an acid. This allows for the analysis of the total number of cations present in a given salt sample by titrating the resulting acid with a base -
 7:35
7:35Quick guide to performing ion exchange chromatography
Quick guide to performing ion exchange chromatographyQuick guide to performing ion exchange chromatography
An breif introduction to cation exchange columns. 1. Draining the equilibration buffer. 0m0s 2. Load and run the Amino Acid (AA) mixture. 1m37s 2. Load Citrate buffer to run AA mixture further into column. 4m24s 3. Summary diagram? 6m56s Note the video was recorded during the class and includes a practical demonstration and a written explanation (pen and paper). These two discussions were spliced together and each cover the same material.
- Adsorption
- Amine
- Amino acid
- Amino acids
- Amphoterism
- Anion
- Biochemistry
- Biomolecule
- Calcium
- Carboxylic acid
- Cation
- Chemical structure
- Chloralkali process
- Chloride
- Chromatography
- Clay
- Complex (chemistry)
- Control rod
- Dealkalization
- Decontamination
- DNA
- Electric charge
- Electrolyte
- Expansive clay
- Filter (water)
- Frank Spedding
- Fuel cell
- Functional group
- Gel
- Glass
- Hafnium
- Humus
- Hydroxide
- Index of refraction
- Inorganic compound
- Ion
- Ion exchange
- Ion exchange resin
- Ion-exchange resin
- Lanthanide
- Lanthanum
- Laundry detergent
- Lutetium
- Magnesium
- Metal
- Mineral
- Molecule
- Montmorillonite
- Neodymium
- Nuclear reactor
- Nuclear reprocessing
- Nuclear weapon
- Organic acid
- Organic base
- Peptide
- Phosphate
- Plutonium
- Polymer
- Potassium
- Power engineering
- Protein
- Proton
- PUREX
- Radioactive waste
- Rare earth element
- Reversible process
- RNA
- Samarium
- Sodium
- Soft water
- Soil
- Soil science
- Solubility
- Solution
- Sorption
- Sulfate
- Thorium
- Uranium
- Water purification
- Water softening
- Waveguide
- Ytterbium
- Zeolite
- Zirconium
-

IonExchange
How ion exchange can be used to soften hard water. -

The Principle of Ion Exchange Chromatography
Download our free 187-page handbook "Ion Exchange Chromatography" http://www.gelifesciences.com/iexhandbook All our handbooks are available here: http://www.gelifesciences.com/handbooks Ion exchange chromatography is one of the most frequently used techniques for purification of biomolecules and separates the molecules according to differences in their net surface charge. This video describes the principles of the technique. -

How an ion exchange water softener works - San Antonio TX
http://texaswatersoftener.com So you know the benefits of a San Antonio water softening system, but you aren't sure how it works. This video explains the ion exchange process along with an animation. Please visit our web site for more information on water softeners and browse our reviews and comparisons. -

Ion Exchange Chromatography
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/ion-exchange-chromatography Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com -

Ion exchange chromatography
-

Ion Exchange Chromatography
Illustration of Ion Exchange Chromatography and how it works on a particle level. IEC is a very powerful and selective separation mode. Biomolecules generally have charged groups on their surfaces. The overall charge changes with the pH of the solution. This is the basis for Ion Exchange Chromatography, in which molecules bind to a charged group of the stationary phase. More information can be found here http://www.separations.eu.tosohbioscience.com/ServiceSupport/TechSupport/ResourceCenter/PrinciplesofChromatography/IonExchange -

Ion exchange chromatography
This chromatography lecture explains about ion exchange chromatography.Web- http://shomusbiology.weebly.com/ Download the study materials here- http://shomusbiology.weebly.com/bio-materials.html This video tutorial explains the mechanisms of ion exchange chromatography and its use in biochemistry -

-

Ion Exchange Chromatography
-

022-Ion Exchange Chromatography
Discussion of principles behind ion exchange chromatography -

Ion Exchange Chromatography - Theory and Principle
http://technologyinscience.blogspot.com/2013/07/an-insight-on-ion-exchange.html The principle of Ion Exchange chromatography separation is the reversible interaction of charged species with the ion exchange matrix. Ion Exchange Chromatography is of Two types: Cation Exchange and Anion Exchange. -

Ion exchange chromatography | cation exchange chromatography and anion exchange chromatography
This comment about the video lecture explains about ion exchange chromatography principle. It also explains the step-by-step process of ion exchange chromatography. That includes the the cation exchange chromatography and anion exchange chromatography. Ion exchange chromatography involves using positive and negative in charge ions to separate charged molecules from a mixture. In the positive this is a problem was in video. For more information, log on to- http://www.shomusbiology.com/ Get Shomu's Biology DVD set here- http://www.shomusbiology.com/dvd-store/ Download the study materials here- http://shomusbiology.com/bio-materials.html Remember Shomu’s Biology is created to spread the knowledge of life science and biology by sharing all this free biology lectures video and animation present... -

Ion Exchange Column
This video demonstrates the use of a cation exchange column to convert a salt to an acid. This allows for the analysis of the total number of cations present in a given salt sample by titrating the resulting acid with a base -

Quick guide to performing ion exchange chromatography
An breif introduction to cation exchange columns. 1. Draining the equilibration buffer. 0m0s 2. Load and run the Amino Acid (AA) mixture. 1m37s 2. Load Citrate buffer to run AA mixture further into column. 4m24s 3. Summary diagram? 6m56s Note the video was recorded during the class and includes a practical demonstration and a written explanation (pen and paper). These two discussions were spliced together and each cover the same material. -

Ion Exchange Lecture - Overview
Ion Exchange Lecture - Overview -

Ion Exchange Process, Fundamentals of Deionization by Ion Exchange, Demineralization Process
For further reading about Ion Exchange Process, Please click on the link given below ... http://vedupro.blogspot.in/2013/05/ion-exchange-process-fundamentals-of.html ION EXCHANGE PROCESS -- DEIONIZATION PROCESS -- DEMINERALIZATION PROCESS Ion exchange resins are insoluble, cross-linked, long chain organic polymers with a micro porous structure, and the "functional groups" attached to the chains are responsible for the ion -- exchange properties. (-COOH, -SO3H etc) are capable of exchange their H plus ions with other cations, which comes in their contact, where as those containing basic functional with other cations, which comes in their contact, where as those containing their anions with other anions, which comes in their contact. The ion exchange resins may be classified as: (1). Catio... -

Ion-exchange resin
An ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small beads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a high surface area. The trapping of ions occurs with the accompanying releasing of other ions; thus the process is called ion-exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate. Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification. In many cases ion-exchange resins were introduced in such processes as a more flexible alternative to the use of natural or artificial zeolites. Also, ion excha... -

ION EXCHANGE (INDIA) LTD- 50 GLORIOUS YEARS
-

ProSystems ION Exchange Process
-

-

-

Chromatography 101: An Introduction to Ion Exchange Chromatography
For more information, visit http://www.bio-rad.com/yt/26/ngc. Shawn Anderson presents an introduction to ion exchange chromatography using Bio-Rad's chromatography instruments, columns, and media to demonstrate the technique. Shawn covers the basic principles of ion exchange chromatography and touches on applications for the separation of proteins and other biomolecules using a variety of anion and cation exchange media. Bio-Rad's Successful Chromatography Webinar series provides a great introduction to the different chromatography methods available for research applications. Most medium pressure chromatography columns work with Bio-Rad's NGC preparative chromatography system. The NGC is designed to rapidly automate the purification of biomolecules. The flexible, modular, and economical d... -

DEAE Sephacel Column: Protein Purification via Ion Exchange
Introduction to Ion Exchange Chromatography for Protein Purification.
IonExchange
- Order: Reorder
- Duration: 9:49
- Updated: 28 Sep 2013
- views: 34836
- published: 28 Sep 2013
- views: 34836
The Principle of Ion Exchange Chromatography
- Order: Reorder
- Duration: 1:41
- Updated: 13 Sep 2012
- views: 177766
- published: 13 Sep 2012
- views: 177766
How an ion exchange water softener works - San Antonio TX
- Order: Reorder
- Duration: 4:51
- Updated: 09 Apr 2013
- views: 62653
- published: 09 Apr 2013
- views: 62653
Ion Exchange Chromatography
- Order: Reorder
- Duration: 10:29
- Updated: 03 Feb 2015
- views: 25027
- published: 03 Feb 2015
- views: 25027
Ion exchange chromatography
- Order: Reorder
- Duration: 6:22
- Updated: 20 Jan 2015
- views: 3126
- published: 20 Jan 2015
- views: 3126
Ion Exchange Chromatography
- Order: Reorder
- Duration: 1:25
- Updated: 29 Sep 2013
- views: 20877
- published: 29 Sep 2013
- views: 20877
Ion exchange chromatography
- Order: Reorder
- Duration: 8:45
- Updated: 27 Oct 2012
- views: 42692
- published: 27 Oct 2012
- views: 42692
Ion Exchange Chromatography
- Order: Reorder
- Duration: 4:23
- Updated: 20 Nov 2011
- views: 17745
Ion Exchange Chromatography
- Order: Reorder
- Duration: 6:46
- Updated: 01 Nov 2012
- views: 13507
- published: 01 Nov 2012
- views: 13507
022-Ion Exchange Chromatography
- Order: Reorder
- Duration: 3:37
- Updated: 10 Jun 2014
- views: 11536
- published: 10 Jun 2014
- views: 11536
Ion Exchange Chromatography - Theory and Principle
- Order: Reorder
- Duration: 6:58
- Updated: 17 May 2014
- views: 44679
- published: 17 May 2014
- views: 44679
Ion exchange chromatography | cation exchange chromatography and anion exchange chromatography
- Order: Reorder
- Duration: 14:59
- Updated: 18 Jan 2016
- views: 307
- published: 18 Jan 2016
- views: 307
Ion Exchange Column
- Order: Reorder
- Duration: 4:25
- Updated: 27 Aug 2009
- views: 47820
- published: 27 Aug 2009
- views: 47820
Quick guide to performing ion exchange chromatography
- Order: Reorder
- Duration: 7:35
- Updated: 02 Apr 2014
- views: 8504
- published: 02 Apr 2014
- views: 8504
Ion Exchange Lecture - Overview
- Order: Reorder
- Duration: 7:16
- Updated: 22 Jul 2011
- views: 15393
Ion Exchange Process, Fundamentals of Deionization by Ion Exchange, Demineralization Process
- Order: Reorder
- Duration: 10:28
- Updated: 13 Dec 2012
- views: 25230
- published: 13 Dec 2012
- views: 25230
Ion-exchange resin
- Order: Reorder
- Duration: 11:19
- Updated: 16 Dec 2015
- views: 662
- published: 16 Dec 2015
- views: 662
ION EXCHANGE (INDIA) LTD- 50 GLORIOUS YEARS
- Order: Reorder
- Duration: 8:25
- Updated: 02 Jun 2015
- views: 203
- published: 02 Jun 2015
- views: 203
ProSystems ION Exchange Process
- Order: Reorder
- Duration: 1:48
- Updated: 29 Jan 2013
- views: 4411
- published: 29 Jan 2013
- views: 4411
PED talk - Soil: Texture, Clay, and Cation Exchange
- Order: Reorder
- Duration: 4:18
- Updated: 05 Dec 2014
- views: 7415
Magnetic Ion Exchange Water System- Virtual Tour
- Order: Reorder
- Duration: 4:24
- Updated: 04 Aug 2009
- views: 8702
Chromatography 101: An Introduction to Ion Exchange Chromatography
- Order: Reorder
- Duration: 33:25
- Updated: 01 Jul 2013
- views: 23862
- published: 01 Jul 2013
- views: 23862
DEAE Sephacel Column: Protein Purification via Ion Exchange
- Order: Reorder
- Duration: 5:52
- Updated: 03 Dec 2014
- views: 2321
- published: 03 Dec 2014
- views: 2321
- Playlist
- Chat
- Playlist
- Chat

IonExchange
- Report rights infringement
- published: 28 Sep 2013
- views: 34836

The Principle of Ion Exchange Chromatography
- Report rights infringement
- published: 13 Sep 2012
- views: 177766

How an ion exchange water softener works - San Antonio TX
- Report rights infringement
- published: 09 Apr 2013
- views: 62653

Ion Exchange Chromatography
- Report rights infringement
- published: 03 Feb 2015
- views: 25027

Ion exchange chromatography
- Report rights infringement
- published: 20 Jan 2015
- views: 3126

Ion Exchange Chromatography
- Report rights infringement
- published: 29 Sep 2013
- views: 20877

Ion exchange chromatography
- Report rights infringement
- published: 27 Oct 2012
- views: 42692

Ion Exchange Chromatography
- Report rights infringement
- published: 20 Nov 2011
- views: 17745

Ion Exchange Chromatography
- Report rights infringement
- published: 01 Nov 2012
- views: 13507

022-Ion Exchange Chromatography
- Report rights infringement
- published: 10 Jun 2014
- views: 11536

Ion Exchange Chromatography - Theory and Principle
- Report rights infringement
- published: 17 May 2014
- views: 44679

Ion exchange chromatography | cation exchange chromatography and anion exchange chromatography
- Report rights infringement
- published: 18 Jan 2016
- views: 307

Ion Exchange Column
- Report rights infringement
- published: 27 Aug 2009
- views: 47820

Quick guide to performing ion exchange chromatography
- Report rights infringement
- published: 02 Apr 2014
- views: 8504
Israeli Military Finds, Destroys Hamas Tunnel Burrowing 30 Meters Into Israeli Territory
Edit WorldNews.com 19 Apr 2016Joe Biden says US feels 'overwhelming frustration' with Israeli government
Edit The Guardian 19 Apr 2016'Elite Overproduction' That Doomed Roman Republic Seen As Threat To U.S. Democracy
Edit WorldNews.com 18 Apr 2016Bernie Sanders backs bill to let Americans sue Saudi Arabia over 9/11 terror attacks
Edit The Independent 19 Apr 2016Boko Haram still a threat months after 'technical victory'
Edit Tampa Bay Online 19 Apr 2016PPMAI approaches PM over anti-circumvention probe on steel
Edit The Times of India 18 Apr 2016Quarterly Activities Report for Period Ending 31 March 2016 (Paladin Energy Ltd)
Edit Public Technologies 18 Apr 2016Paladin Energy Limited: Quarterly Activities Report for the Period Ending 31 March 2016
Edit Stockhouse 18 Apr 2016AU Environmental Sustainability Symposium Concludes with Back-to-Back Events (Ashland University)
Edit Public Technologies 06 Apr 2016A patented nanotechnology for clean drinking water (Lehigh University)
Edit Public Technologies 05 Apr 2016Scientists develop green technology for water purification
Edit The Times of India 03 Apr 2016Affymetrix, Sigma-Aldrich, BASF - Research and Markets
Edit Business Wire 31 Mar 2016Des Moines Fights to Keep Its Water Clean
Edit Bloomberg 31 Mar 2016In lead crises, a maker of water-filtration pitchers sees opportunity clearly
Edit Philadelphia Daily News 27 Mar 2016Calcium gives liquid batteries a surprising boost
Edit Yahoo Daily News 25 Mar 2016New lithium battery ditches solvents, reaches supercapacitor rates
Edit Ars Technica 23 Mar 2016New lithium battery ditches solvents, reaches supercapacitor rates [US]
Edit Ars Technica 22 Mar 2016Fuel-cell technology provides new approach to energy-efficient heating and refrigeration (University of Delaware)
Edit Public Technologies 16 Mar 2016- 1
- 2
- 3
- 4
- 5
- Next page »







