7:59
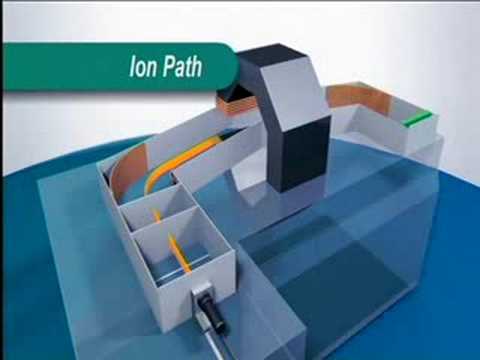
Mass Spectrometry MS
An education video on Mass Spectrometry using a magnetic sector instrument from the Royal ...
published: 27 Sep 2008
author: wwwRSCorg
Mass Spectrometry MS
Mass Spectrometry MS
An education video on Mass Spectrometry using a magnetic sector instrument from the Royal Society of Chemistry. From the Modern Instrumental Techniques for s...- published: 27 Sep 2008
- views: 307286
- author: wwwRSCorg
4:52

Simple explanation of the Mass Spectrometer.
http://www.franklychemistry.co.uk is my YouTube website. Thanks for visiting! This short f...
published: 08 Aug 2012
author: Franklychemistry
Simple explanation of the Mass Spectrometer.
Simple explanation of the Mass Spectrometer.
http://www.franklychemistry.co.uk is my YouTube website. Thanks for visiting! This short flash animation video outlines the basic principles of a Mass Spectr...- published: 08 Aug 2012
- views: 23557
- author: Franklychemistry
8:20

Mass Spectrometry
009 - Mass Spectrometry In this video Paul Andersen explains how a spectrometer was used t...
published: 08 Aug 2013
author: bozemanbiology
Mass Spectrometry
Mass Spectrometry
009 - Mass Spectrometry In this video Paul Andersen explains how a spectrometer was used to identify the presence of isotopes. This modified Dalton's origina...- published: 08 Aug 2013
- views: 813
- author: bozemanbiology
10:48

Introduction to mass spectrometry (1)
Organic chemistry: Mass spectrometry. Molecular/parent ion; base peak. Carbon-13; bromine ...
published: 31 Aug 2009
author: freelanceteach
Introduction to mass spectrometry (1)
Introduction to mass spectrometry (1)
Organic chemistry: Mass spectrometry. Molecular/parent ion; base peak. Carbon-13; bromine and chlorine isotopes. Fragmentation and substitution This is a rec...- published: 31 Aug 2009
- views: 156553
- author: freelanceteach
2:44

2.2 The Mass Specrtometer
The Mass Spectrometer. How it works, and a couple simple related exercisese....
published: 11 Apr 2012
author: Evagating
2.2 The Mass Specrtometer
2.2 The Mass Specrtometer
The Mass Spectrometer. How it works, and a couple simple related exercisese.- published: 11 Apr 2012
- views: 602
- author: Evagating
5:27

2.2.1 Describe and explain the operation of a mass spectrometer IB Chemistry SL
This machine measures the relative mass of atoms (after converting them into ions first) t...
published: 03 Sep 2012
author: Richard Thornley
2.2.1 Describe and explain the operation of a mass spectrometer IB Chemistry SL
2.2.1 Describe and explain the operation of a mass spectrometer IB Chemistry SL
This machine measures the relative mass of atoms (after converting them into ions first) to C-12. You need to learn the 5 parts and briefly explain them. You...- published: 03 Sep 2012
- views: 5912
- author: Richard Thornley
24:06

Mass spectrometry part 1 : introduction
For more information, log on to-
http://shomusbiology.weebly.com/
Download the study mater...
published: 20 Sep 2013
Mass spectrometry part 1 : introduction
Mass spectrometry part 1 : introduction
For more information, log on to- http://shomusbiology.weebly.com/ Download the study materials here- http://shomusbiology.weebly.com/bio-materials.html Mass spectrometry (MS) is an analytical technique that produces spectra (singular spectrum) of the masses of the molecules comprising a sample of material. The spectra are used to determine the elemental composition of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules, such as peptides and other chemical compounds. Mass spectrometry works by ionizing chemical compounds to generate charged molecules or molecule fragments and measuring their mass-to-charge ratios.[1] In a typical MS procedure, a sample, which may be solid, liquid, or gas, is ionized. The ions are separated according to their mass-to-charge ratio.[1] The ions are detected by a mechanism capable of detecting charged particles. Signal processing results are displayed as spectra of the relative abundance of ions as a function of the mass-to-charge ratio. The atoms or molecules can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern. A mass spectrometer consists of three components: an ion source, a mass analyzer, and a detector.[2] The ionizer converts a portion of the sample into ions. There is a wide variety of ionization techniques, depending on the phase (solid, liquid, gas) of the sample and the efficiency of various ionization mechanisms for the unknown species. An extraction system removes ions from the sample, which are then trajected through the mass analyzer and onto the detector. The differences in masses of the fragments allows the mass analyzer to sort the ions by their mass-to-charge ratio. The detector measures the value of an indicator quantity and thus provides data for calculating the abundances of each ion present. Some detectors also give spatial information, e.g. a multichannel plate. Mass spectrometry has both qualitative and quantitative uses. These include identifying unknown compounds, determining the isotopic composition of elements in a molecule, and determining the structure of a compound by observing its fragmentation. Other uses include quantifying the amount of a compound in a sample or studying the fundamentals of gas phase ion chemistry (the chemistry of ions and neutrals in a vacuum). MS is now in very common use in analytical laboratories that study physical, chemical, or biological properties of a great variety of compounds. As an analytical technique it possesses distinct advantages such as: 1. Increased sensitivity over most other analytical techniques because the analyzer, as a mass-charge filter, reduces background interference 2. Excellent specificity from characteristic fragmentation patterns to identify unknowns or confirm the presence of suspected compounds. 3. Information about molecular weight. 4. Information about the isotopic abundance of elements. 5. Temporally resolved chemical data. A few of the disadvantages of the method is that often fails to distinguish between optical and geometrical isomers and the positions of substituent in o-, m- and p- positions in an aromatic ring. Also, its scope is limited in identifying hydrocarbons that produce similar fragmented ions.[3]- published: 20 Sep 2013
- views: 19
2:26

NASA | The Molecule Dissector - Mass Spectrometry
What do you do if you have a sample from another planet, and you want to find out if it co...
published: 22 Jul 2010
author: NASAexplorer
NASA | The Molecule Dissector - Mass Spectrometry
NASA | The Molecule Dissector - Mass Spectrometry
What do you do if you have a sample from another planet, and you want to find out if it contains a certain molecule...maybe even one that will reveal that th...- published: 22 Jul 2010
- views: 30708
- author: NASAexplorer
55:22

Lecture 4. Mass Spectrometry: Theory, Instrumentation, and Techniques
This video is part of a 28-lecture graduate-level course titled "Organic Spectroscopy" tau...
published: 30 Nov 2011
author: UCITLTC
Lecture 4. Mass Spectrometry: Theory, Instrumentation, and Techniques
Lecture 4. Mass Spectrometry: Theory, Instrumentation, and Techniques
This video is part of a 28-lecture graduate-level course titled "Organic Spectroscopy" taught at UC Irvine by Professor James S. Nowick. The course covers in...- published: 30 Nov 2011
- views: 28955
- author: UCITLTC
8:55

Mass spectrometry
Easy way to learn Mass Spectroscopy. Organic chemistry....
published: 18 Jan 2013
author: Abhishiek Setia
Mass spectrometry
Mass spectrometry
Easy way to learn Mass Spectroscopy. Organic chemistry.- published: 18 Jan 2013
- views: 3891
- author: Abhishiek Setia
15:00

Introduction to Mass Spectrometry
PDF: http://www.mediafire.com/?b49d624iub6k22i....
published: 12 May 2012
author: Alex Baklajian
Introduction to Mass Spectrometry
Introduction to Mass Spectrometry
PDF: http://www.mediafire.com/?b49d624iub6k22i.- published: 12 May 2012
- views: 14407
- author: Alex Baklajian
1:20

Mass Spectrometry: General Principles
Mass spectrometry (also known as mass spectroscopy and MS) is an analytical technique used...
published: 09 Nov 2013
Mass Spectrometry: General Principles
Mass Spectrometry: General Principles
Mass spectrometry (also known as mass spectroscopy and MS) is an analytical technique used to measure the mass-to-charge ratio of ions. It is used to measure elemental composition of sample, determ...- published: 09 Nov 2013
- views: 8
9:08

The Mass Spectrometer
One of a series of 7 physics videos made for 1st year 'A' level students by the Hertfordsh...
published: 12 Mar 2008
author: HuwGS
The Mass Spectrometer
The Mass Spectrometer
One of a series of 7 physics videos made for 1st year 'A' level students by the Hertfordshire Science Centre. (1988) Use the search tag 'HertsScienceCentre' ...- published: 12 Mar 2008
- views: 53741
- author: HuwGS
8:41

Introduction to Ionization and Fragmentation in Mass Spectrometry
Professor Davis uses pentane to demonstrate the electron impact ionization process and fra...
published: 14 Jul 2013
author: ChemSurvival
Introduction to Ionization and Fragmentation in Mass Spectrometry
Introduction to Ionization and Fragmentation in Mass Spectrometry
Professor Davis uses pentane to demonstrate the electron impact ionization process and fragmentation patterns in mass spectrometry of alkanes.- published: 14 Jul 2013
- views: 81
- author: ChemSurvival
Youtube results:
65:21

Mass Spectrometry Lecture.
http://www.franklychemistry.co.uk is my YouTube website. Thanks for visiting! This is the ...
published: 13 Apr 2012
author: Franklychemistry
Mass Spectrometry Lecture.
Mass Spectrometry Lecture.
http://www.franklychemistry.co.uk is my YouTube website. Thanks for visiting! This is the first of a pair of videos intended to help senior school chemistry ...- published: 13 Apr 2012
- views: 24794
- author: Franklychemistry
4:09

6500 Series Accurate-Mass Q-TOF LC/MS Systems: How it works
View 6500 Series Accurate-Mass Q-TOF LC/MS systems from the inside. This animation follows...
published: 28 Sep 2009
author: AgilentLife
6500 Series Accurate-Mass Q-TOF LC/MS Systems: How it works
6500 Series Accurate-Mass Q-TOF LC/MS Systems: How it works
View 6500 Series Accurate-Mass Q-TOF LC/MS systems from the inside. This animation follows sample molecules from ionization through mass filtering, fragmenta...- published: 28 Sep 2009
- views: 28030
- author: AgilentLife
35:53

Mass spectrometry part 3 : protein analysis (step by step process)
For more information, log on to-
http://shomusbiology.weebly.com/
Download the study mater...
published: 21 Sep 2013
Mass spectrometry part 3 : protein analysis (step by step process)
Mass spectrometry part 3 : protein analysis (step by step process)
For more information, log on to- http://shomusbiology.weebly.com/ Download the study materials here- http://shomusbiology.weebly.com/bio-materials.html Mass spectrometry (MS) is an analytical technique that produces spectra (singular spectrum) of the masses of the molecules comprising a sample of material. The spectra are used to determine the elemental composition of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules, such as peptides and other chemical compounds. Mass spectrometry works by ionizing chemical compounds to generate charged molecules or molecule fragments and measuring their mass-to-charge ratios.[1] In a typical MS procedure, a sample, which may be solid, liquid, or gas, is ionized. The ions are separated according to their mass-to-charge ratio.[1] The ions are detected by a mechanism capable of detecting charged particles. Signal processing results are displayed as spectra of the relative abundance of ions as a function of the mass-to-charge ratio. The atoms or molecules can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern. A mass spectrometer consists of three components: an ion source, a mass analyzer, and a detector.[2] The ionizer converts a portion of the sample into ions. There is a wide variety of ionization techniques, depending on the phase (solid, liquid, gas) of the sample and the efficiency of various ionization mechanisms for the unknown species. An extraction system removes ions from the sample, which are then trajected through the mass analyzer and onto the detector. The differences in masses of the fragments allows the mass analyzer to sort the ions by their mass-to-charge ratio. The detector measures the value of an indicator quantity and thus provides data for calculating the abundances of each ion present. Some detectors also give spatial information, e.g. a multichannel plate. Mass spectrometry has both qualitative and quantitative uses. These include identifying unknown compounds, determining the isotopic composition of elements in a molecule, and determining the structure of a compound by observing its fragmentation. Other uses include quantifying the amount of a compound in a sample or studying the fundamentals of gas phase ion chemistry (the chemistry of ions and neutrals in a vacuum). MS is now in very common use in analytical laboratories that study physical, chemical, or biological properties of a great variety of compounds. As an analytical technique it possesses distinct advantages such as: 1. Increased sensitivity over most other analytical techniques because the analyzer, as a mass-charge filter, reduces background interference 2. Excellent specificity from characteristic fragmentation patterns to identify unknowns or confirm the presence of suspected compounds. 3. Information about molecular weight. 4. Information about the isotopic abundance of elements. 5. Temporally resolved chemical data. A few of the disadvantages of the method is that often fails to distinguish between optical and geometrical isomers and the positions of substituent in o-, m- and p- positions in an aromatic ring. Also, its scope is limited in identifying hydrocarbons that produce similar fragmented ions.[3]- published: 21 Sep 2013
- views: 3










