Pentazocine
 |
|
|---|---|
| Systematic (IUPAC) name | |
| (2RS,6RS,11RS)-6,11-dimethyl-3-(3-methylbut-2-en-1-yl)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocin-8-ol or 2-dimethylallyl-5,9-dimethyl-2'-hydroxybenzomorphan |
|
| Clinical data | |
| Pregnancy cat. | C/D (U.S.) |
| Legal status | Controlled (S8) (AU) Schedule IV (U.S.) III international |
| Routes | Oral, IV, IM |
| Pharmacokinetic data | |
| Bioavailability | ~20% orally |
| Metabolism | Hepatic |
| Half-life | 2 to 3 hours |
| Excretion | Renal |
| Identifiers | |
| CAS number | 359-83-1 |
| ATC code | N02AD01 |
| PubChem | CID 441278 |
| IUPHAR ligand | 1606 |
| DrugBank | DB00652 |
| ChemSpider | 390041 |
| UNII | RP4A60D26L |
| KEGG | D00498 |
| ChEMBL | CHEMBL560 |
| Chemical data | |
| Formula | C19H27NO |
| Mol. mass | 285.424 g/mol |
|
|
|
|
| |
|
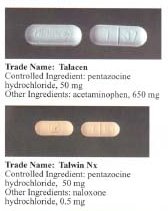
Pentazocine is a synthetically prepared prototypical mixed agonist–antagonist narcotic (opioid analgesic) drug of the benzomorphan class of opioids used to treat moderate to moderately severe pain. Pentazocine is sold under several brand names, such as Fortral,Sosegon, Talwin NX (with the μ-antagonist naloxone, will cause withdrawal in opioid dependent persons on injection), Talwin, Talwin PX (without naloxone), Fortwin (Lactate injectable form) and Talacen (with acetaminophen). This compound may exist as one of two enantiomers, named (+)-pentazocine and (-)-pentazocine. (-)-pentazocine is a κ-opioid receptor agonist, while (+)-pentazocine is not, instead displaying a ten-fold greater affinity for the σ receptor. Talwin PX is the main pentazocine pharmaceutical in Canada, where laws and regulations prohibit the addition of naloxone to the formulation for non-therapeutic purposes[citation needed]. Related drugs include phenazocine, dezocine, cyclazocine and several chemicals used in research on the central nervous system.
Contents |
[edit] Therapeutic uses
Pentazocine is used to treat moderate to severe pain.
[edit] Administration
Pentazocine is administered by subcutaneous (rarely), intramuscular, and intravenous injection as the lactate.[1] It is also available in 50 mg tablets with naloxone added.[2]
[edit] Adverse effects
Side effects are similar to those of morphine, but pentazocine may be more likely to cause hallucinations and other psychotomimetic effects; cardiovascular effects make it unsuitable for use in myocardial infarction. Unlike morphine, its respiratory depressant action is subject to a "ceiling" effect. 30 milligrams of pentazocine has the same pain relieving capacity as 10 milligrams of morphine.[1]
[edit] Tissue damage at injection sites
Severe injection site necrosis and sepsis has occurred (sometime requiring amputation of limb) with multiple injection of pentazocine lactate. In addition, animal studies have demonstrated that Pentazocine is tolerated less well subcutaneously than intramuscularly.[1]
[edit] Illicit use
In the 1970s, recreational drug users discovered that combining pentazocine with tripelennamine (a first-generation ethylenediamine antihistamine most commonly dispensed under the brand names Pelamine and Pyribenzamine) produced a euphoric sensation. Since tripelennamine tablets are typically blue in color and brand-name Pentazocine is known as Talwin (hence "Ts"), the pentazocine/tripelennamine combination acquired the slang name Ts and blues. After health-care professionals and drug-enforcement officials became aware of this scenario, the mu-opioid-antagonist naloxone was added to oral preparations containing pentazocine to prevent injection misuse,[2] and the reported incidence of its misuse has declined precipitously since.
[edit] Legal status
Pentazocine is still classified in Schedule IV under the Controlled Substances Act in the United States, even with the addition of Naloxone. Internationally, pentazocine is a Schedule III drug under the Convention on Psychotropic Substances.[3]
[edit] History
Pentazocine was developed by the Sterling Drug Company, Sterling-Winthrop Research Institute, of Rensselaer, New York. It was approved by the Food and Drug Administration in June 1967 after being favorably reviewed following testing on 12,000 patients in the United States. The analgesic compound was first made at Sterling in 1958. U.S. testing was conducted between 1961 - 1967. By mid 1967 Pentazocine was already being sold in Mexico, England, and Argentina, under different trade names.[4]
[edit] References
- ^ a b c "TALWIN (pentazocine lactate) injection, solution". DailyMed. National Institute of Health. Retrieved 2011-12-10.
- ^ a b "Pentazocine and Naloxone tablets". DailyMed. National Institute of Health. Retrieved 2011-12-10.
- ^ "List of psychotropic substances under international control". Green List - Annex to the annual statistical report on psychotropic substances (form P) (23rd ed.). International Narcotics Control Board. August 2003.
- ^ Pain-Killing Drug Approved By F.D.A., New York Times, June 27, 1967, pg. 41.
[edit] External links
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||