Breast cancer (malignant breast neoplasm) is a type of cancer originating from breast tissue, most commonly from the inner lining of milk ducts or the lobules that supply the ducts with milk.[1] Cancers originating from ducts are known as ductal carcinomas; those originating from lobules are known as lobular carcinomas. Breast cancer is a disease of humans and other mammals; while the overwhelming majority of cases in humans are women, men can sometimes also develop breast cancer.[2]
The size, stage, rate of growth, and other characteristics of the tumor determine the kinds of treatment. Treatment may include surgery, drugs (hormonal therapy and chemotherapy), radiation and/or immunotherapy.[3] Surgical removal of the tumor provides the single largest benefit, with surgery alone being capable of producing a cure in many cases. To somewhat increase the likelihood of long-term disease-free survival, several chemotherapy regimens are commonly given in addition to surgery. Most forms of chemotherapy kill cells that are dividing rapidly anywhere in the body, and as a result cause temporary hair loss and digestive disturbances. Radiation is indicated especially after breast conserving surgery and substantially improves local relapse rates and in many circumstances also overall survival.[4] Some breast cancers are sensitive to hormones such as estrogen and/or progesterone, which makes it possible to treat them by blocking the effects of these hormones.
Worldwide, breast cancer comprises 22.9% of all cancers (excluding non-melanoma skin cancers) in women.[5] In 2008, breast cancer caused 458,503 deaths worldwide (13.7% of cancer deaths in women).[5] Breast cancer is more than 100 times more common in women than breast cancer in men, although males tend to have poorer outcomes due to delays in diagnosis.[6][7]
Prognosis and survival rates vary greatly depending on cancer type, staging and treatment, and geographical location of the patient. Survival rates in the Western World are very good,[6] for instance, overall, more than 8 out of 10 women (84%) in England that are diagnosed with the disease survive it for at least 5 years.[8] In the developing countries, however, survival rates are much poorer.

Early signs of breast cancer

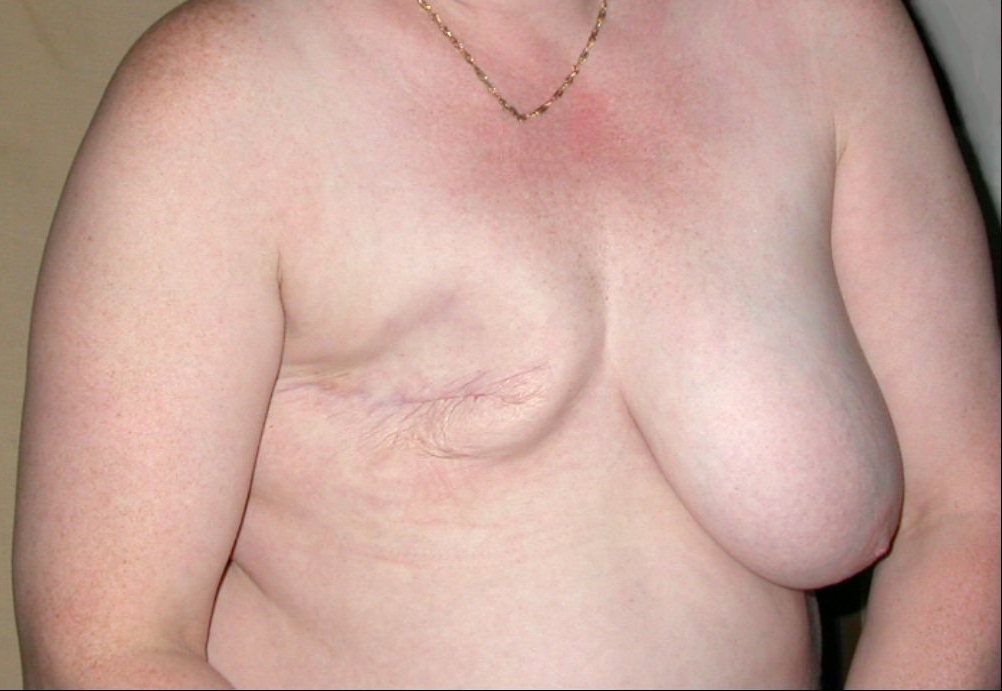
Breast cancer showing an inverted nipple, lump and skin dimpling.
The first noticeable symptom of breast cancer is typically a lump that feels different from the rest of the breast tissue. More than 80% of breast cancer cases are discovered when the woman feels a lump.[9] The earliest breast cancers are detected by a mammogram.[10] Lumps found in lymph nodes located in the armpits[9] can also indicate breast cancer.
Indications of breast cancer other than a lump may include thickening different from the other breast tissue, one breast becoming larger or lower, a nipple changing position or shape or becoming inverted, skin puckering or dimpling, a rash on or around a nipple, discharge from nipple/s, constant pain in part of the breast or armpit, and swelling beneath the armpit or around the collarbone.[11] Pain ("mastodynia") is an unreliable tool in determining the presence or absence of breast cancer, but may be indicative of other breast health issues.[9][10][12]
Inflammatory breast cancer is a particular type of breast cancer which can pose a substantial diagnostic challenge. Symptoms may resemble a breast inflammation and may include itching, pain, swelling, nipple inversion, warmth and redness throughout the breast, as well as an orange-peel texture to the skin referred to as peau d'orange;[9] the absence of a discernible lump delays detection dangerously.
Another reported symptom complex of breast cancer is Paget's disease of the breast. This syndrome presents as eczematoid skin changes such as redness and mild flaking of the nipple skin. As Paget's advances, symptoms may include tingling, itching, increased sensitivity, burning, and pain. There may also be discharge from the nipple. Approximately half of women diagnosed with Paget's also have a lump in the breast.[13]
In rare cases, what initially appears as a fibroadenoma (hard movable lump) could in fact be a phyllodes tumor. Phyllodes tumors are formed within the stroma (connective tissue) of the breast and contain glandular as well as stromal tissue. Phyllodes tumors are not staged in the usual sense; they are classified on the basis of their appearance under the microscope as benign, borderline, or malignant.[14]
Occasionally, breast cancer presents as metastatic disease, that is, cancer that has spread beyond the original organ. Metastatic breast cancer will cause symptoms that depend on the location of metastasis. Common sites of metastasis include bone, liver, lung and brain.[15] Unexplained weight loss can occasionally herald an occult breast cancer, as can symptoms of fevers or chills. Bone or joint pains can sometimes be manifestations of metastatic breast cancer, as can jaundice or neurological symptoms. These symptoms are called non-specific, meaning they could be manifestations of many other illnesses.[16]
Most symptoms of breast disorders, including most lumps, do not turn out to represent underlying breast cancer. Less than 20% of lumps for example are cancer[17] and benign breast diseases such as mastitis and fibroadenoma of the breast are more common causes of breast disorder symptoms. Nevertheless, the appearance of a new symptom should be taken seriously by both patients and their doctors, because of the possibility of an underlying breast cancer at almost any age.[18]
The primary risk factors for breast cancer are female sex,[19] age,[20] lack of childbearing or breastfeeding,[21] higher hormone levels,[22][23] race, economic status and dietary iodine deficiency.[24][25][26]
Smoking tobacco may increase the risk of breast cancer with the greater the amount of smoking and the earlier in life smoking begins the higher the risk.[27][28]
In a study of attributable risk and epidemiological factors published in 1995, later age at first birth and not having children accounted for 29.5% of U.S. breast cancer cases, family history of breast cancer accounted for 9.1% and factors correlated with higher income contributed 18.9% of cases.[29] Attempts to explain the increased incidence (but lower mortality) correlated with higher income include epidemiologic observations such as lower birth rates correlated with higher income and better education, possible overdiagnosis and overtreatment because of better access to breast cancer screening, and the postulation of as yet unexplained lifestyle and dietary factors correlated with higher income. In more recent years, research has indicated the impact of diet and other behaviors on breast cancer. These additional risk factors include a high-fat diet,[30] alcohol intake,[31] obesity,[32] and environmental factors such as tobacco use, radiation,[33] endocrine disruptors and shiftwork.[34] Although the radiation from mammography is a low dose, the cumulative effect can cause cancer.[35] [36]
In addition to the risk factors specified above, demographic and medical risk factors include:
- Personal history of breast cancer: A woman who had breast cancer in one breast has an increased risk of getting a second breast cancer.
- Family history: A woman's risk of breast cancer is higher if her mother, sister, or daughter had breast cancer, the risk becomes significant if at least two close relatives had breast or ovarian cancer. The risk is higher if her family member got breast cancer before age 40. An Australian study found that having other relatives with breast cancer (in either her mother's or father's family) may also increase a woman's risk of breast cancer and other forms of cancer, including brain and lung cancers.[37]
- Certain breast changes: Atypical hyperplasia and lobular carcinoma in situ found in benign breast conditions such as fibrocystic breast changes are correlated with an increased breast cancer risk.
Those with a normal body mass index at age 20 who gained weight as they aged had nearly double the risk of developing breast cancer after menopause in comparison to women who maintained their weight. The average 60-year-old woman's risk of developing breast cancer by age 65 is about 2 percent; her lifetime risk is 13 percent.[38]
The genes associated with hereditary breast-ovarian cancer syndromes usually increase the risk slightly or moderately; the exception is women and men who are carriers of BRCA mutations. These people have a very high lifetime risk for breast and ovarian cancer, depending on the portion of the proteins where the mutation occurs. Instead of a 12 percent (one in eight) lifetime risk of breast cancer, women with one of these genes have a risk of approximately 60 percent.[39] Approximately two percent of the female population carries the BRCA1 or BRCA2 gene mutation. The vast majority of breast cancer cases (greater than 80%) are considered non-hereditary sporadic breast cancer or estrogen positive breast cancer cases. A woman's probability of developing one of these types of breast cancer can be predicted using predictive medicine.
There is an association between oral contraceptives and the development of premenopausal breast cancer.[40] Whether or not this association is causal is debated and if there is indeed a link the absolute effect is small.[41] The abortion–breast cancer hypothesis posits that induced abortion increases the risk of developing breast cancer.[42] This hypothesis has been the subject of extensive scientific inquiry which has concluded that abortion does not cause breast cancer.[43][44][45]
Main article:
Carcinogenesis

Overview of signal transduction pathways involved in
apoptosis. Mutations leading to loss of apoptosis can lead to tumorigenesis.
Breast cancer, like other cancers, occurs because of an interaction between the environment and a defective gene. Normal cells divide as many times as needed and stop. They attach to other cells and stay in place in tissues. Cells become cancerous when mutations destroy their ability to stop dividing, to attach to other cells and to stay where they belong.
Normal cells will commit cell suicide (apoptosis) when they are no longer needed. Until then, they are protected from cell suicide by several protein clusters and pathways. One of the protective pathways is the PI3K/AKT pathway; another is the RAS/MEK/ERK pathway. Sometimes the genes along these protective pathways are mutated in a way that turns them permanently "on", rendering the cell incapable of committing suicide when it is no longer needed. This is one of the steps that causes cancer in combination with other mutations. Normally, the PTEN protein turns off the PI3K/AKT pathway when the cell is ready for cell suicide. In some breast cancers, the gene for the PTEN protein is mutated, so the PI3K/AKT pathway is stuck in the "on" position, and the cancer cell does not commit suicide.[46]
Mutations that can lead to breast cancer have been experimentally linked to estrogen exposure.[47]
Failure of immune surveillance, the removal of malignant cells throughout one's life by the immune system.[48] Abnormal growth factor signaling in the interaction between stromal cells and epithelial cells can facilitate malignant cell growth.[49][50] In breast adipose tissue, overexpression of leptin leads to increased cell proliferation and cancer.[51]
In the United States, 10 to 20 percent of patients with breast cancer and patients with ovarian cancer have a first- or second-degree relative with one of these diseases. The familial tendency to develop these cancers is called hereditary breast—ovarian cancer syndrome. The best known of these, the BRCA mutations, confer a lifetime risk of breast cancer of between 60 and 85 percent and a lifetime risk of ovarian cancer of between 15 and 40 percent. Some mutations associated with cancer, such as p53, BRCA1 and BRCA2, occur in mechanisms to correct errors in DNA. These mutations are either inherited or acquired after birth. Presumably, they allow further mutations, which allow uncontrolled division, lack of attachment, and metastasis to distant organs.[33][52]However there is strong evidence of residual risk variation that goes well beyond hereditary BRCA gene mutations between carrier families. This is caused by unobserved risk factors.[53] This implicates environmental and other causes as triggers for breast cancers. The inherited mutation in BRCA1 or BRCA2 genes can interfere with repair of DNA cross links and DNA double strand breaks (known functions of the encoded protein) [54] Because of this repair deficit, risks from carcinogenic chemicals and ionizing radiation can increase [55]These carcinogens cause DNA damage such as DNA cross links and double strand breaks that often require repairs by pathways containing BRCA1 and BRCA2.[56][57] But it is these repair pathways that can be crippled by inherited mutation. There is evidence that cancer risks increase in mutation carriers exposed to such opportunistic carcinogens.[58] Thus risks for cancers may be reduced by avoiding or compensating for carcinogens that exploit the inherited BRCA gene deficiency [59] However, mutations in BRCA genes account for only 2 to 3 percent of all breast cancers.[60] About half of hereditary breast–ovarian cancer syndromes involve unknown genes.
Most types of breast cancer are easy to diagnose by microscopic analysis of the biopsy. There are however, rarer types of breast cancer that require specialized lab exams.
While screening techniques are useful in determining the possibility of cancer, a further testing is necessary to confirm whether a lump detected on screening is cancer, as opposed to a benign alternative such as a simple cyst.
Very often the results of noninvasive examination, mammography and additional tests that are performed in special circumstances such as ultrasound or MR imaging are sufficient to warrant excisional biopsy as the definitive diagnostic and curative method.
Both mammography and clinical breast exam, also used for screening, can indicate an approximate likelihood that a lump is cancer, and may also detect some other lesions.[61] When the tests are inconclusive Fine Needle Aspiration and Cytology (FNAC) may be used. FNAC may be done in a GP's office using local anaesthetic if required, involves attempting to extract a small portion of fluid from the lump. Clear fluid makes the lump highly unlikely to be cancerous, but bloody fluid may be sent off for inspection under a microscope for cancerous cells. Together, these three tools can be used to diagnose breast cancer with a good degree of accuracy.
Other options for biopsy include core biopsy, where a section of the breast lump is removed, and an excisional biopsy, where the entire lump is removed.
In addition vacuum-assisted breast biopsy (VAB) may help diagnose breast cancer among patients with a mammographically detected breast in women.[62]
-
Excised human breast tissue, showing an irregular, dense, white stellate area of cancer 2 cm in diameter, within yellow fatty tissue.
-
High grade invasive ductal carcinoma, with minimal tubule formation, marked pleomorphism, and prominent mitoses, 40x field.
-
Micrograph showing a lymph node invaded by ductal breast carcinoma and with extranodal extension of tumour.
-
Neuropilin-2 expression in normal breast and breast carcinoma tissue.
-
F-18 FDG PET/CT: Metastasis of a mamma carcinoma in the right scapula
Breast cancers are classified by several grading systems. Each of these influences the prognosis and can affect treatment response. Description of a breast cancer optimally includes all of these factors.
- Histopathology. Breast cancer is usually classified primarily by its histological appearance. Most breast cancers are derived from the epithelium lining the ducts or lobules, and these cancers are classified as ductal or lobular carcinoma. Carcinoma in situ is growth of low grade cancerous or precancerous cells within a particular tissue compartment such as the mammary duct without invasion of the surrounding tissue. In contrast, invasive carcinoma does not confine itself to the initial tissue compartment.[63]
- Grade. Grading compares the appearance of the breast cancer cells to the appearance of normal breast tissue. Normal cells in an organ like the breast become differentiated, meaning that they take on specific shapes and forms that reflect their function as part of that organ. Cancerous cells lose that differentiation. In cancer, the cells that would normally line up in an orderly way to make up the milk ducts become disorganized. Cell division becomes uncontrolled. Cell nuclei become less uniform. Pathologists describe cells as well differentiated (low grade), moderately differentiated (intermediate grade), and poorly differentiated (high grade) as the cells progressively lose the features seen in normal breast cells. Poorly differentiated cancers have a worse prognosis.
- Stage. Breast cancer staging using the TNM system is based on the size of thetumor (T), whether or not the tumor has spread to the lymph nodes (N) in the armpits, and whether the tumor has metastasized (M) (i.e. spread to a more distant part of the body). Larger size, nodal spread, and metastasis have a larger stage number and a worse prognosis.
The main stages are:
- Receptor status. Breast cancer cells have receptors on their surface and in their cytoplasm and nucleus. Chemical messengers such as hormones bind to receptors, and this causes changes in the cell. Breast cancer cells may or may not have three important receptors: estrogen receptor (ER), progesterone receptor (PR), and HER2.
ER+ cancer cells depend on estrogen for their growth, so they can be treated with drugs to block estrogen effects (e.g. tamoxifen), and generally have a better prognosis. HER2+ breast cancer had a worse prognosis,[64] but HER2+ cancer cells respond to drugs such as the monoclonal antibody trastuzumab (in combination with conventional chemotherapy), and this has improved the prognosis significantly.[65] Cells with none of these receptors are called triple negative although they frequently express receptors for other hormones such as androgen receptor and prolactin receptor.
- DNA assays. DNA testing of various types including DNA microarrays have compared normal cells to breast cancer cells. The specific changes in a particular breast cancer can be used to classify the cancer in several ways, and may assist in choosing the most effective treatment for that DNA type.
Exercise may decrease breast cancer risk.[66] Also avoiding alcohol and obesity. Prophylactic bilateral mastectomy may be considered in patients with BRCA1 and BRCA2 mutations.[67][68] A 2007 report concluded that women can somewhat reduce their risk by maintaining a healthy weight, drinking less alcohol, being physically active and breastfeeding their children.[69] Some carcinogens are known to take advantage of deficiencies in processes that depend on normal BRCA1 and BRCA2 function. Avoiding these known carcinogens may reduce risks for BRCA1/2 mutation carriers [70]
The World Cancer Research Fund estimated that 38% of breast cancer cases in the US are preventable through reducing alcohol intake, increasing physical activity levels and maintaining a healthy weight.[71] It also estimated that 42% of breast cancer cases in the UK could be prevented in this way, as well as 28% in Brazil and 20% in China.
Breast cancer screening refers to testing otherwise-healthy women for breast cancer in an attempt to achieve an earlier diagnosis. The assumption is that early detection will improve outcomes. A number of screening test have been employed including: clinical and self breast exams, mammography, genetic screening, ultrasound, and magnetic resonance imaging.
A clinical or self breast exam involves feeling the breast for lumps or other abnormalities. Research evidence does not support the effectiveness of either type of breast exam, because by the time a lump is large enough to be found it is likely to have been growing for several years and will soon be large enough to be found without an exam.[72] Mammographic screening for breast cancer uses x-rays to examine the breast for any uncharacteristic masses or lumps. The Cochrane collaboration in 2011 concluded that mammograms reduce mortality from breast cancer by 15 percent but also result in unnecessary surgery and anxiety, resulting in their view that it is not clear whether mammography screening does more good or harm.[73] Many national organizations recommend regular mammography, nevertheless. For the average woman, the U.S. Preventive Services Task Force recommends mammography every two years in women between the ages of 50 and 74.[74] The Task Force points out that in addition to unnecessary surgery and anxiety, the risks of more frequent mammograms include a small but significant increase in breast cancer induced by radiation.[75][55]
In women at high risk, such as those with a strong family history of cancer, mammography screening is recommended at an earlier age and additional testing may include genetic screening that tests for the BRCA genes and / or magnetic resonance imaging. Molecular breast imaging is currently under study and may also be an alternative.[76]
Breast cancer is usually treated with surgery and then possibly with chemotherapy or radiation, or both. A multidisciplinary approach is preferable.[77] Hormone positive cancers are treated with long term hormone blocking therapy. Treatments are given with increasing aggressiveness according to the prognosis and risk of recurrence.
- Stage 1 cancers (and DCIS) have an excellent prognosis and are generally treated with lumpectomy and sometimes radiation.[78] HER2+ cancers should be treated with the trastuzumab (Herceptin) regime.[79] Chemotherapy is uncommon for other types of stage 1 cancers.
- Stage 2 and 3 cancers with a progressively poorer prognosis and greater risk of recurrence are generally treated with surgery (lumpectomy or mastectomy with or without lymph node removal), chemotherapy (plus trastuzumab for HER2+ cancers) and sometimes radiation (particularly following large cancers, multiple positive nodes or lumpectomy).
- Stage 4, metastatic cancer, (i.e. spread to distant sites) has poor prognosis and is managed by various combination of all treatments from surgery, radiation, chemotherapy and targeted therapies. 10 year survival rate is 5% without treatment and 10% with optimal treatment.[80]
Surgery involves the physical removal of the tumor, typically along with some of the surrounding tissue and frequently sentinel node biopsy.
Standard surgeries include:
If the patient desires, then breast reconstruction surgery, a type of cosmetic surgery, may be performed to create an aesthetic appearance. In other cases, women use breast prostheses to simulate a breast under clothing, or choose a flat chest.
Drugs used after and in addition to surgery are called adjuvant therapy. Chemotherapy or other types of therapy prior to surgery are called neoadjuvant therapy.
There are currently three main groups of medications used for adjuvant breast cancer treatment: hormone blocking therapy, chemotherapy, and monoclonal antibodies.
Hormone blocking therapy: Some breast cancers require estrogen to continue growing. They can be identified by the presence of estrogen receptors (ER+) and progesterone receptors (PR+) on their surface (sometimes referred to together as hormone receptors). These ER+ cancers can be treated with drugs that either block the receptors, e.g. tamoxifen (Nolvadex), or alternatively block the production of estrogen with an aromatase inhibitor, e.g. anastrozole (Arimidex)[81] or letrozole (Femara). Aromatase inhibitors, however, are only suitable for post-menopausal patients. This is because the active aromatase in postmenopausal women is different from the prevalent form in premenopausal women, and therefore these agents are ineffective in inhibiting the predominant aromatase of premenopausal women.[82]
Chemotherapy: Predominately used for stage 2–4 disease, being particularly beneficial in estrogen receptor-negative (ER-) disease. They are given in combinations, usually for 3–6 months. One of the most common treatments is cyclophosphamide plus doxorubicin (Adriamycin), known as AC. Most chemotherapy medications work by destroying fast-growing and/or fast-replicating cancer cells either by causing DNA damage upon replication or other mechanisms; these drugs also damage fast-growing normal cells where they cause serious side effects. Damage to the heart muscle is the most dangerous complication of doxorubicin. Sometimes a taxane drug, such as docetaxel, is added, and the regime is then known as CAT; taxane attacks the microtubules in cancer cells. Another common treatment, which produces equivalent results, is cyclophosphamide, methotrexate, and fluorouracil (CMF). (Chemotherapy can literally refer to any drug, but it is usually used to refer to traditional non-hormone treatments for cancer.)[citation needed]
Monoclonal antibodies: Trastuzumab (Herceptin), a monoclonal antibody to HER2, has improved the 5 year disease free survival of stage 1–3 HER2+ breast cancers to about 87% (overall survival 95%).[83] Trastuzumab, however, is expensive, and approximately 2% of patients suffer significant heart damage.[84] Other monoclonal antibodies are also undergoing clinical trials. Trastuzumab is only effective in patients with the HER2 mutation. Between 25 and thirty percent of breast cancers have an amplification of the HER2 gene or overexpression of its protein product.[85] This receptor is normally stimulated by a growth factor which causes the cell to divide; in the absence of the growth factor, the cell will normally stop growing. Overexpression of this receptor in breast cancer is associated with increased disease recurrence and worse prognosis.
Aspirin may reduce mortality from breast cancer.[86]
Radiotherapy is given after surgery to the region of the tumor bed and regional lymph nodes, to destroy microscopic tumor cells that may have escaped surgery. It may also have a beneficial effect on tumor microenvironment.[87][88] Radiation therapy can be delivered as external beam radiotherapy or as brachytherapy (internal radiotherapy). Conventionally radiotherapy is given after the operation for breast cancer. Radiation can also be given at the time of operation on the breast cancer- intraoperatively. The largest randomised trial to test this approach was the TAR-GIT-A Trial[89] which found that targeted intraoperative radiotherapy was equally effective at 4-years as the usual several weeks' of whole breast external beam radiotherapy.[90] Radiation can reduce the risk of recurrence by 50–66% (1/2 – 2/3 reduction of risk) when delivered in the correct dose[91] and is considered essential when breast cancer is treated by removing only the lump (Lumpectomy or Wide local excision).

An example of recurrent breast cancer
A prognosis is a prediction of outcome and the probability of progression-free survival (PFS) or disease-free survival (DFS). These predictions are based on experience with breast cancer patients with similar classification. A prognosis is an estimate, as patients with the same classification will survive a different amount of time, and classifications are not always precise. Survival is usually calculated as an average number of months (or years) that 50% of patients survive, or the percentage of patients that are alive after 1, 5, 15, and 20 years. Prognosis is important for treatment decisions because patients with a good prognosis are usually offered less invasive treatments, such as lumpectomy and radiation or hormone therapy, while patients with poor prognosis are usually offered more aggressive treatment, such as more extensive mastectomy and one or more chemotherapy drugs.
Prognostic factors are reflected in the classification scheme for breast cancer including stage, (i.e., tumor size, location, whether disease has spread to lymph nodes and other parts of the body), grade, recurrence of the disease, and the age and health of the patient. The Nottingham Prognostic Index is a commonly used prognostic tool.
The stage of the breast cancer is the most important component of traditional classification methods of breast cancer, because it has a greater effect on the prognosis than the other considerations. Staging takes into consideration size, local involvement, lymph node status and whether metastatic disease is present. The higher the stage at diagnosis, the poorer the prognosis. The stage is raised by the invasiveness of disease to lymph nodes, chest wall, skin or beyond, and the aggressiveness of the cancer cells. The stage is lowered by the presence of cancer-free zones and close-to-normal cell behaviour (grading). Size is not a factor in staging unless the cancer is invasive. For example, Ductal Carcinoma In Situ (DCIS) involving the entire breast will still be stage zero and consequently an excellent prognosis with a 10yr disease free survival of about 98%.[92]
The breast cancer grade is assessed by comparison of the breast cancer cells to normal breast cells. The closer to normal the cancer cells are, the slower their growth and the better the prognosis. If cells are not well differentiated, they will appear immature, will divide more rapidly, and will tend to spread. Well differentiated is given a grade of 1, moderate is grade 2, while poor or undifferentiated is given a higher grade of 3 or 4 (depending upon the scale used). The most widely used grading system is the Nottingham scheme;[93] details are provided in the discussion of breast cancer grade.
The presence of estrogen and progesterone receptors in the cancer cell is important in guiding treatment. Those who do not test positive for these specific receptors will not be able to respond to hormone therapy, and this can affect their chance of survival depending upon what treatment options remain, the exact type of the cancer, and how advanced the disease is.
In addition to hormone receptors, there are other cell surface proteins that may affect prognosis and treatment. HER2 status directs the course of treatment. Patients whose cancer cells are positive for HER2 have more aggressive disease and may be treated with the 'targeted therapy', trastuzumab (Herceptin), a monoclonal antibody that targets this protein and improves the prognosis significantly.
Younger women tend to have a poorer prognosis than post-menopausal women due to several factors. Their breasts are active with their cycles, they may be nursing infants, and may be unaware of changes in their breasts. Therefore, younger women are usually at a more advanced stage when diagnosed. There may also be biologic factors contributing to a higher risk of disease recurrence for younger women with breast cancer.[94]
The emotional impact of cancer diagnosis, symptoms, treatment, and related issues can be severe. Most larger hospitals are associated with cancer support groups which provide a supportive environment to help patients cope and gain perspective from cancer survivors. Online cancer support groups are also very beneficial to cancer patients, especially in dealing with uncertainty and body-image problems inherent in cancer treatment.
Not all breast cancer patients experience their illness in the same manner. Factors such as age can have a significant impact on the way a patient copes with a breast cancer diagnosis. Premenopausal women with estrogen-receptor positive breast cancer must confront the issues of early menopause induced by many of the chemotherapy regimens used to treat their breast cancer, especially those that use hormones to counteract ovarian function.[95]
On the other hand, a small 2007 study conducted by researchers at the College of Public Health of the University of Georgia suggested a need for greater attention to promoting functioning and psychological well-being among older cancer survivors, even when they may not have obvious cancer-related medical complications.[96] The study found that older breast cancer survivors showed multiple indications of decrements in their health-related quality of life, and lower psychosocial well-being than a comparison group. Survivors reported no more depressive symptoms or anxious mood than the comparison group, however, they did score lower in measures of positive psychosocial well-being, and reported more depressed mood and days affected by fatigue. As the incidence of breast cancer in women over 50 rises and survival rates increase, breast cancer is increasingly becoming a geriatric issue that warrants both further research and the expansion of specialized cancer support services tailored for specific age groups.[96]

Age-standardized death from breast cancer per 100,000 inhabitants in 2004.
[97]
no data
<2
2–4
4–6
6–8
8–10
10–12
|
12–14
14–16
16–18
18–20
20–22
>22
|
Worldwide, breast cancer is the most common invasive cancer in women. (The most common form of cancer is non-invasive non-melanoma skin cancer; non-invasive cancers are generally easily cured, cause very few deaths, and are routinely excluded from cancer statistics.) Breast cancer comprises 22.9% of invasive cancers in women[5] and 16% of all female cancers.[98]
In 2008, breast cancer caused 458,503 deaths worldwide (13.7% of cancer deaths in women and 6.0% of all cancer deaths for men and women together).[5] Lung cancer, the second most common cause of cancer-related death in women, caused 12.8% of cancer deaths in women (18.2% of all cancer deaths for men and women together).[5]
The incidence of breast cancer varies greatly around the world: it is lowest in less-developed countries and greatest in the more-developed countries. In the twelve world regions, the annual age-standardized incidence rates per 100,000 women are as follows: in Eastern Asia, 18; South Central Asia, 22; sub-Saharan Africa, 22; South-Eastern Asia, 26; North Africa and Western Asia, 28; South and Central America, 42; Eastern Europe, 49; Southern Europe, 56; Northern Europe, 73; Oceania, 74; Western Europe, 78; and in North America, 90.[99]
The number of cases worldwide has significantly increased since the 1970s, a phenomenon partly attributed to the modern lifestyles.[100][101] Breast cancer is strongly related to age with only 5% of all breast cancers occurring in women under 40 years old.[102]

Breast cancer surgery in 18th century
Because of its visibility, breast cancer was the form of cancer most often described in ancient documents.[103] Because autopsies were rare, cancers of the internal organs were essentially invisible to ancient medicine. Breast cancer, however, could be felt through the skin, and in its advanced state often developed into fungating lesions: the tumor would become necrotic (die from the inside, causing the tumor to appear to break up) and ulcerate through the skin, weeping fetid, dark fluid.[103]
The oldest description of cancer was discovered in Egypt and dates back to approximately 1600 BC. The Edwin Smith Papyrus describes 8 cases of tumors or ulcers of the breast that were treated by cauterization. The writing says about the disease, "There is no treatment."[104] For centuries, physicians described similar cases in their practises, with the same conclusion. Ancient medicine, from the time of the Greeks through the 17th century, was based on humoralism, and thus believed that breast cancer was generally caused by imbalances in the fundamental fluids that controlled the body, especially an excess of black bile.[105] Alternatively, patients often saw it as divine punishment.[106] In the 18th century, a wide variety of medical explanations were proposed, including a lack of sexual activity, too much sexual activity, physical injuries to the breast, curdled breast milk, and various forms of lymphatic blockages, either internal or due to restrictive clothing.[105][107] In the 19th century, the Scottish surgeon John Rodman said that fear of cancer caused cancer, and that this anxiety, learned by example from the mother, accounted for breast cancer's tendency to run in families.[107]
Although breast cancer was known in ancient times, it was uncommon until the 19th century, when improvements in sanitation and control of deadly infectious diseases resulted in dramatic increases in lifespan. Previously, most women had died too young to have developed breast cancer.[107] Additionally, early and frequent childbearing and breastfeeding probably reduced the rate of breast cancer development in those women who did survive to middle age.[107]
Because ancient medicine believed that the cause was systemic, rather than local, and because surgery carried a high mortality rate, the preferred treatments tended to be pharmacological rather than surgical. Herbal and mineral preparations, especially involving the poison arsenic, were relatively common.
Mastectomy for breast cancer was performed at least as early as AD 548, when it was proposed by the court physician Aetios of Amida to Theodora.[103] It was not until doctors achieved greater understanding of the circulatory system in the 17th century that they could link breast cancer's spread to the lymph nodes in the armpit. The French surgeon Jean Louis Petit (1674–1750) and later the Scottish surgeon Benjamin Bell (1749–1806) were the first to remove the lymph nodes, breast tissue, and underlying chest muscle.[108]
Their successful work was carried on by William Stewart Halsted who started performing radical mastectomies in 1882, helped greatly by advances in general surgical technology, such as aseptic technique and anesthesia. The Halsted radical mastectomy often involved removing both breasts, associated lymph nodes, and the underlying chest muscles. This often led to long-term pain and disability, but was seen as necessary in order to prevent the cancer from recurring.[109] Before the advent of the Halsted radical mastectomy, 20-year survival rates were only 10%; Halsted's surgery raised that rate to 50%.[110] Extending Halsted's work, Jerome Urban promoted superradical mastectomies, taking even more tissue, until 1963, when the ten-year survival rates proved equal to the less-damaging radical mastectomy.[109]
Radical mastectomies remained the standard of care in America until the 1970s, but in Europe, breast-sparing procedures, often followed radiation therapy, were generally adopted in the 1950s.[109] One reason for this striking difference in approach may be the structure of the medical professions: European surgeons, descended from the barber surgeon, were held in less esteem than physicians; in America, the surgeon was the king of the medical profession.[109] Additionally, there were far more European women surgeons: Less than one percent of American surgical oncologists were female, but some European breast cancer wards boasted a medical staff that was half female.[109] American health insurance companies also paid surgeons more to perform radical mastectomies than they did to perform more intricate breast-sparing surgeries.[109]
Breast cancer staging systems were developed in the 1920s and 1930s.[109]
During the 1970s, a new understanding of metastasis led to perceiving cancer as a systemic illness as well as a localized one, and more sparing procedures were developed that proved equally effective. Modern chemotherapy developed after World War II.[111]
The French surgeon Bernard Peyrilhe (1737–1804) realized the first experimental transmission of cancer by injecting extracts of breast cancer into an animal.
Prominent women who died of breast cancer include Anne of Austria, the mother of Louis XIV of France; Mary Washington, mother of George, and Rachel Carson, the environmentalist.[112]
The first case-controlled study on breast cancer epidemiology was done by Janet Lane-Claypon, who published a comparative study in 1926 of 500 breast cancer cases and 500 control patients of the same background and lifestyle for the British Ministry of Health.[113]
In the 1980s and 1990s, thousands of women who had successfully completed standard treatment then demanded and received high-dose bone marrow transplants, thinking this would lead to better long-term survival. However, it proved completely ineffective, and 15–20% of women died because of the brutal treatment.[114]
The 1995 reports from the Nurses' Health Study and the 2002 conclusions of the Women's Health Initiative trial conclusively proved that hormone replacement therapy significantly increased the incidence of breast cancer.[114]
Before the 20th century, breast cancer was feared and discussed in hushed tones, as if it were shameful. As little could be safely done with primitive surgical techniques, women tended to suffer silently rather than seeking care. When surgery advanced, and long-term survival rates improved, women began raising awareness of the disease and the possibility of successful treatment. The "Women's Field Army", run by the American Society for the Control of Cancer (later the American Cancer Society) during the 1930s and 1940s was one of the first organized campaigns. In 1952, the first peer-to-peer support group, called "Reach to Recovery", began providing post-mastectomy, in-hospital visits from women who had survived breast cancer.[115]
The breast cancer movement of the 1980s and 1990s developed out of the larger feminist movements and women's health movement of the 20th century.[116] This series of political and educational campaigns, partly inspired by the politically and socially effective AIDS awareness campaigns, resulted in the widespread acceptance of second opinions before surgery, less invasive surgical procedures, support groups, and other advances in patient care.[117]

The
pink ribbon is a symbol to show support for breast cancer awareness
Main article:
Pink ribbonA pink ribbon is the most prominent symbol of breast cancer awareness. Pink ribbons, which can be made inexpensively, are sometimes sold as fundraisers, much like poppies on Remembrance Day. They may be worn to honor those who have been diagnosed with breast cancer, or to identify products that the manufacturer would like to sell to consumers that are interested in breast cancer—usually white, middle-aged, middle-class and upper-class, educated women.[118]
The pink ribbon is associated with individual generosity, faith in scientific progress, and a "can-do" attitude. It encourages consumers to focus on the emotionally appealing ultimate vision of a cure for breast cancer, rather than on the fraught path between current knowledge and any future cures.[119]
Wearing or displaying a pink ribbon has been criticized by the opponents of this practice as a kind of slacktivism, because it has no practical positive effect and as hypocrisy among those who wear the pink ribbon to show good will towards women with breast cancer, but then oppose these women's practical goals, like patient rights and anti-pollution legislation.[120][121] Critics say that the feel-good nature of pink ribbons and pink consumption distracts society from the lack of progress on preventing and curing breast cancer.[122] It is also criticized for reinforcing gender stereotypes and objectifying women and their breasts.[123] Breast Cancer Action launched the "Think Before You Pink" campaign, and charged that companies have co-opted the pink campaign to promote products that encourage breast cancer, such as high-fat Kentucky Fried Chicken and alcohol.[124]
Breast cancer culture, or pink ribbon culture, is the set of activities, attitudes, and values that surround and shape breast cancer in public. The dominant values are selflessness, cheerfulness, unity, and optimism. Appearing to have suffered bravely is the passport into the culture.
The woman with breast cancer is given a cultural template that constrains her emotional and social responses into a socially acceptable discourse: She is to use the emotional trauma of being diagnosed with breast cancer and the suffering of extended treatment to transform herself into a stronger, happier and more sensitive person who is grateful for the opportunity to become a better person. Breast cancer thereby becomes a rite of passage rather than a disease.[125] To fit into this mold, the woman with breast cancer needs to normalize and feminize her appearance, and minimize the disruption that her health issues cause anyone else. Anger, sadness and negativity must be silenced.[125]
As with most cultural models, people who conform to the model are given social status, in this case as cancer survivors. Women who reject the model are shunned, punished and shamed.[125]
The culture is criticized for treating adult women like little girls, as evidenced by "baby" toys such as pink teddy bears given to adult women.[125]
The primary purposes or goals of breast cancer culture are to maintain breast cancer's dominance as the preëminent women's health issue, to promote the appearance that society is "doing something" effective about breast cancer, and to sustain and expand the social, political, and financial power of breast cancer activists.[126]
Compared to other diseases or other cancers, breast cancer receives a disproportionate share of resources and attention. In 2001 MP Ian Gibson, chairman of the House of Commons of the United Kingdom all party group on cancer stated "The treatment has been skewed by the lobbying, there is no doubt about that. Breast cancer sufferers get better treatment in terms of bed spaces, facilities and doctors and nurses."[127] Breast cancer also receives significantly more media coverage than other, equally prevalent cancers, with a study by Prostate Coalition showing 2.6 breast cancer stories for each one covering cancer of the prostate.[128] Ultimately there is a concern that favouring sufferers of breast cancer with disproportionate funding and research on their behalf may well be costing lives elsewhere.[127] Partly because of its relatively high prevalence and long-term survival rates, research is biased towards breast cancer. Some subjects, such as cancer-related fatigue, have been studied in little except women with breast cancer.
One result of breast cancer's high visibility is that most women significantly overestimate their personal risk of dying from it. Misleading statistics, such as the claim that one in eight women will be diagnosed with breast cancer during their lives—a claim that depends on the patently unrealistic assumption that no woman will die of any other disease before the age of 95[129]—obscure the reality, which is that about ten times as many women will die from heart disease or stroke than from breast cancer.[130]
The emphasis on breast cancer screening may be harming women by subjecting them to unnecessary radiation, biopsies, and surgery. One-third of diagnosed breast cancers might recede on their own.[131] Screening mammography efficiently finds non-life-threatening, asymptomatic breast cancers and pre-cancers, even while overlooking serious cancers. According to H. Gilbert Welch of the Dartmouth Institute for Health Policy and Clinical Practice, research on screening mammography has taken the "brain-dead approach that says the best test is the one that finds the most cancers" rather than the one that finds dangerous cancers.[131]
Several historical paintings show anomalies that have been interpreted as visible evidence of breast cancer; retrospective diagnoses are discussed in the medical literature. Possible signs of breast cancer such as a typical lump, differences in breast size or shape and the peau d'orange skin texture can be found for example in works by Raphael, Rembrandt and Rubens.[103][132][133][134]
The paintings and the historical context do not give enough information to conclude whether or not the visible changes are really signs of breast cancer[135] and alternative explanations such as tuberculous mastitis or a chronic lactational breast abscess need to be considered.[136]
- Examples of breast cancer signs in art
-
Raffaelo Sanzio (1483–1520): Portrait of a young woman (La Fornarina)
-
-
-
-
-
Cancers found during or shortly after pregnancy appear at approximately the same rate as other cancers in women of a similar age. As a result, breast cancer is one of the more common cancers found during pregnancy, although it is still rare, because only about 1 in 1,000 pregnant women experience any sort of cancer.[137]
Diagnosing a new cancer in a pregnant woman is difficult, in part because any symptoms are commonly assumed to be a normal discomfort associated with pregnancy.[137] As a result, cancer is typically discovered at a somewhat later stage than average in many pregnant or recently pregnant women. Some imaging procedures, such as MRIs (magnetic resonance imaging), CT scans, ultrasounds, and mammograms with fetal shielding are considered safe during pregnancy; some others, such as PET scans are not.[137]
Treatment is generally the same as for non-pregnant women.[137] However, radiation is normally avoided during pregnancy, especially if the fetal dose might exceed 100 cGy. In some cases, some or all treatments are postponed until after birth if the cancer is diagnosed late in the pregnancy. Early deliveries to speed the start of treatment are not uncommon. Surgery is generally considered safe during pregnancy, but some other treatments, especially certain chemotherapy drugs given during the first trimester, increase the risk of birth defects and pregnancy loss (spontaneous abortions and stillbirths).[137] Elective abortions are not required and do not improve the likelihood of the mother surviving or being cured.[137]
Radiation treatments may interfere with the mother's ability to breastfeed her baby because it reduces the ability of that breast to produce milk and increases the risk of mastitis. Also, when chemotherapy is being given after birth, many of the drugs pass through breast milk to the baby, which could harm the baby.[137]
A considerable part of the current knowledge on breast carcinomas is based on in vivo and in vitro studies performed with breast cancer cell (BCC) lines. These provide an unlimited source of homogenous self-replicating material, free of contaminating stromal cells, and often easily cultured in simple standard media. The first line described, BT-20, was established in 1958. Since then, and despite sustained work in this area, the number of permanent lines obtained has been strikingly low (about 100). Indeed, attempts to culture BCC from primary tumors have been largely unsuccessful. This poor efficiency was often due to technical difficulties associated with the extraction of viable tumor cells from their surrounding stroma. Most of the available BCC lines issued from metastatic tumors, mainly from pleural effusions. Effusions provided generally large numbers of dissociated, viable tumor cells with little or no contamination by fibroblasts and other tumor stroma cells. Many of the currently used BCC lines were established in the late 1970s. A very few of them, namely MCF-7, T-47D, and MDA-MB-231, account for more than two-thirds of all abstracts reporting studies on mentioned BCC lines, as concluded from a Medline-based survey.
Treatments are constantly evaluated in randomized, controlled trials, to evaluate and compare individual drugs, combinations of drugs, and surgical and radiation techniques. The latest research is reported annually at scientific meetings such as that of the American Society of Clinical Oncology, San Antonio Breast Cancer Symposium,[138] and the St. Gallen Oncology Conference in St. Gallen, Switzerland.[139] These studies are reviewed by professional societies and other organizations, and formulated into guidelines for specific treatment groups and risk category.
- List of cell lines
Mainly based on Lacroix and Leclercq (2004).[140] For more data on the nature of TP53 mutations in breast cancer cell lines, see Lacroix et al. (2006).[141]
- ^ Sariego, J. (2010). "Breast cancer in the young patient". The American surgeon 76 (12): 1397–1401. PMID 21265355. edit
- ^ US NIH: Male Breast Cancer
- ^ Florescu, A.; Amir, E.; Bouganim, N.; Clemons, M. (2011). "Immune therapy for breast cancer in 2010—hype or hope?". Current Oncology 18 (1): e9–e18. PMC 3031364. PMID 21331271. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3031364.
- ^ Buchholz TA (January 2009). "Radiation therapy for early-stage breast cancer after breast-conserving surgery". N. Engl. J. Med. 360 (1): 63–70. DOI:10.1056/NEJMct0803525. PMID 19118305.
- ^ a b c d e "World Cancer Report". International Agency for Research on Cancer. 2008. http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900. Retrieved 2011-02-26. (cancer statistics often exclude non-melanoma skin cancers such as basal cell carcinoma which though very common are rarely fatal)
- ^ a b "World Cancer Report". International Agency for Research on Cancer. 2008. http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf. Retrieved 2011-02-26.
- ^ "Male Breast Cancer Treatment". National Cancer Institute. 2011. http://www.cancer.gov/cancertopics/pdq/treatment/malebreast/HealthProfessional. Retrieved 2011-02-26.
- ^ ONS, Cancer Survival in England, patients diagnosed 2004-08, followed up to 2009. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-216670
- ^ a b c d Merck Manual of Diagnosis and Therapy (February 2003). "Breast Disorders: Breast Cancer". http://www.merckmanuals.com/home/womens_health_issues/breast_disorders/breast_cancer.html. Retrieved 2008-02-05.
- ^ a b American Cancer Society (2007). "Cancer Facts & Figures 2007" (PDF). Archived from the original on April 10, 2007. http://web.archive.org/web/20070410025934/http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. Retrieved 2007-04-26.
- ^ Watson, Max (2008). "Assessment of suspected cancer". InnoAiT 1 (2): 94–107. DOI:10.1093/innovait/inn001. http://rcgp-innovait.oxfordjournals.org.
- ^ eMedicine (August 23, 2006). "Breast Cancer Evaluation". http://www.emedicine.com/med/TOPIC3287.HTM. Retrieved 2008-02-05.
- ^ National Cancer Institute (June 27, 2005). "Paget's Disease of the Nipple: Questions and Answers". http://www.cancer.gov/cancertopics/factsheet/Sites-Types/pagets-breast. Retrieved 2008-02-06.
- ^ answers.com. "Oncology Encyclopedia: Cystosarcoma Phyllodes". http://www.answers.com/topic/phyllodes-tumor. Retrieved 2010-08-10.
- ^ Lacroix M (December 2006). "Significance, detection and markers of disseminated breast cancer cells". Endocrine-related Cancer 13 (4): 1033–67. DOI:10.1677/ERC-06-0001. PMID 17158753.
- ^ National Cancer Institute (September 1, 2004). "Metastatic Cancer: Questions and Answers". http://www.cancer.gov/cancertopics/factsheet/Sites-Types/metastatic. Retrieved 2008-02-06.
- ^ McCann], [executive publisher, Judith A. Shilling (2008). Nursing.. Ambler, PA: Lippincott Williams & Wilkins. pp. 99. ISBN 978-1-58255-668-0. http://books.google.ca/books?id=PcARTQwHLpIC&pg=PA99.
- ^ Merck Manual of Diagnosis and Therapy (February 2003). "Breast Disorders: Overview of Breast Disorders". http://www.merckmanuals.com/home/womens_health_issues/breast_disorders/overview_of_breast_disorders.html. Retrieved 2008-02-05.
- ^ Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN (July 2004). "Breast carcinoma in men: a population-based study". Cancer 101 (1): 51–7. DOI:10.1002/cncr.20312. PMID 15221988.
- ^ "Breast Cancer Risk Factors". 2008-11-25. http://www.breastcancer.org/symptoms/understand_bc/risk/factors.jsp. Retrieved 2009-11-10.
- ^ Collaborative Group on Hormonal Factors in Breast Cancer (August 2002). "Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease.". Lancet 360 (9328): 187–95. DOI:10.1016/S0140-6736(02)09454-0. PMID 12133652.
- ^ Yager JD; Davidson NE (2006). "Estrogen carcinogenesis in breast cancer". New Engl J Med 354 (3): 270–82. DOI:10.1056/NEJMra050776. PMID 16421368.
- ^ Santoro, E., DeSoto, M., and Hong Lee, J (February 2009). "Hormone Therapy and Menopause". National Research Center for Women & Families. http://www.center4research.org/2010/03/hormone-therapy-and-menopause/.
- ^ Venturi, S. (2001). "Is there a role for iodine in breast diseases?". The Breast 10 (5): 379–382. DOI:10.1054/brst.2000.0267. PMID 14965610. edit
- ^ Aceves, C.; Anguiano, B.; Delgado, G. (2005). "Is iodine a gatekeeper of the integrity of the mammary gland?". Journal of Mammary Gland Biology and Neoplasia 10 (2): 189–196. DOI:10.1007/s10911-005-5401-5. PMID 16025225. edit
- ^ Stoddard Fr, 2nd; Brooks, AD; Eskin, BA; Johannes, GJ (2008). "Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine". International journal of medical sciences 5 (4): 189–96. PMC 2452979. PMID 18645607. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2452979. edit
- ^ Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB (January 2011). "Cigarette smoking and the incidence of breast cancer". Arch. Intern. Med. 171 (2): 125–33. DOI:10.1001/archinternmed.2010.503. PMID 21263102.
- ^ Johnson, KC; Miller, AB, Collishaw, NE, Palmer, JR, Hammond, SK, Salmon, AG, Cantor, KP, Miller, MD, Boyd, NF, Millar, J, Turcotte, F (2011 Jan). "Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009).". Tobacco control 20 (1): e2. DOI:10.1136/tc.2010.035931. PMID 21148114.
- ^ Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN (November 1995). "Proportion of breast cancer cases in the United States explained by well-established risk factors". Journal of the National Cancer Institute 87 (22): 1681–5. DOI:10.1093/jnci/87.22.1681. PMID 7473816.
- ^ Chlebowski RT, Blackburn GL, Thomson CA, et al. (December 2006). "Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study". Journal of the National Cancer Institute 98 (24): 1767–76. DOI:10.1093/jnci/djj494. PMID 17179478.
- ^ Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J (August 2006). "The burden of cancer attributable to alcohol drinking". International Journal of Cancer 119 (4): 884–7. DOI:10.1002/ijc.21903. PMID 16557583.
- ^ BBC report Weight link to breast cancer risk
- ^ a b American Cancer Society (2005). "Breast Cancer Facts & Figures 2005–2006" (PDF). Archived from the original on June 13, 2007. http://web.archive.org/web/20070613192148/http://www.cancer.org/downloads/STT/CAFF2005BrFacspdf2005.pdf. Retrieved 2007-04-26.
- ^ WHO international Agency for Research on CancerPress Release No. 180, December 2007.
- ^ Feig SA, Hendrick RE (1997). "Radiation risk from screening mammography of women aged 40–49 years.". J Natl Cancer Inst Monogr 22 (22): 119–24. PMID 9709287.
- ^ "2009 Update: When Should Women Start Regular Mammograms? 40? 50? And How Often is "Regular"?". National Research Center for Women & Families. November 2009. http://www.stopcancerfund.org/posts/211.
- ^ Medew, Julia (30 September 2010). "Study finds big risk of cancer in the family". Sydney Morning Herald. http://www.smh.com.au/lifestyle/wellbeing/study-finds-big-risk-of-cancer-in-the-family-20100929-15xin.html. Retrieved 30 September 2010.
- ^ "Gain in Body Mass Index Increases Postmenopausal Breast Cancer Risk". National Cancer Institute. http://benchmarks.cancer.gov/2010/04/gain-in-body-mass-index-increases-postmenopausal-breast-cancer-risk. Retrieved 2010-04-26.
- ^ National Cancer Institute BRCA1 and BRCA2: Cancer Risk and Genetic Testing
- ^ Kahlenborn, C; Modugno, F, Potter, DM, Severs, WB (2006 Oct). "Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis.". Mayo Clinic proceedings. Mayo Clinic 81 (10): 1290–302. DOI:10.4065/81.10.1290. PMID 17036554.
- ^ Veljković, M; Veljković, S (2010 Sep-Oct). "[The risk of breast cervical, endometrial and ovarian cancer in oral contraceptive users].". Medicinski pregled 63 (9-10): 657–61. PMID 21446095.
- ^ Russo J, Russo I (1980). "Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence". Am J Pathol 100 (2): 505–506. PMC 1903536. PMID 6773421. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1903536. "In contrast, abortion is associated with increased risk of carcinomas of the breast. The explanation for these epidemiologic findings is not known, but the parallelism between the DMBA-induced rat mammary carcinoma model and the human situation is striking. [...] Abortion would interrupt this process, leaving in the gland undifferentiated structures like those observed in the rat mammary gland, which could render the gland again susceptible to carcinogenesis."
- ^ "WHO – Induced abortion does not increase breast cancer risk". who.int. Archived from the original on 13 January 2011. http://www.who.int/mediacentre/factsheets/fs240/en/index.html. Retrieved 2011-01-11.
- ^ "Abortion, Miscarriage, and Breast Cancer Risk". National Cancer Institute. Archived from the original on 21 December 2010. http://www.cancer.gov/cancertopics/factsheet/risk/abortion-miscarriage. Retrieved 2011-01-11.
- ^ "Politics & Science – Investigating the State of Science Under the Bush Administration". oversight.house.gov. Archived from the original on March 27, 2008. http://web.archive.org/web/20080327055020/http://oversight.house.gov/features/politics_and_science/example_breast_cancer.htm. Retrieved 2008-04-14.
- ^ "32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium". Sunday Morning Year-End Review. Dec. 14, 2009. http://www.sabcs.org/Newsletter/Docs/SABCS_2009_Issue5.pdf.
- ^ Cavalieri E, Chakravarti D, Guttenplan J, et al. (August 2006). "Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention". Biochimica et Biophysica Acta 1766 (1): 63–78. DOI:10.1016/j.bbcan.2006.03.001. PMID 16675129.
- ^ Farlex (2005). "Immunological Surveilliance". The Free Dictionary. http://medical-dictionary.thefreedictionary.com/immunological+surveillance. Retrieved 2008-02-10.
- ^ Haslam SZ, Woodward TL. (June 2003). "Host microenvironment in breast cancer development: epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland.". Breast Cancer Res. 5 (4): 208–15. DOI:10.1186/bcr615. PMC 165024. PMID 12817994. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=165024.
- ^ Wiseman BS, Werb Z (May 2002). "Stromal effects on mammary gland development and breast cancer". Science 296 (5570): 1046–9. DOI:10.1126/science.1067431. PMC 2788989. PMID 12004111. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2788989.
- ^ Jardé T, Perrier S, Vasson MP, Caldefie-Chézet F (January 2011). "Molecular mechanisms of leptin and adiponectin in breast cancer". Eur. J. Cancer 47 (1): 33–43. DOI:10.1016/j.ejca.2010.09.005. PMID 20889333.
- ^ Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF (1 October 1999). "A systematic review of genetic polymorphisms and breast cancer risk". Cancer Epidemiology, Biomarkers & Prevention 8 (10): 843–54. PMID 10548311. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=10548311.
- ^ Begg CB, Haile RW, Borg A, et al. (January 2008). "Variation of breast cancer risk among BRCA1/2 carriers". JAMA 299 (2): 194–201. DOI:10.1001/jama.2007.55-a. PMC 2714486. PMID 18182601. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=18182601.
- ^ Patel KJ, Yu VP, Lee H, et al. (February 1998). "Involvement of Brca2 in DNA repair". Mol. Cell 1 (3): 347–57. DOI:10.1016/S1097-2765(00)80035-0. PMID 9660919. http://linkinghub.elsevier.com/retrieve/pii/S1097-2765(00)80035-0.
- ^ a b Friedenson B (March 2000). "Is mammography indicated for women with defective BRCA genes? Implications of recent scientific advances for the diagnosis, treatment, and prevention of hereditary breast cancer". MedGenMed 2 (1): E9. PMID 11104455. http://www.medscape.com/Medscape/GeneralMedicine/journal/2000/v02.n02/mgm0309.frie/mgm0309.frie-01.html.
- ^ Marietta C, Thompson LH, Lamerdin JE, Brooks PJ (May 2009). "Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: implications for alcohol-related carcinogenesis". Mutat. Res. 664 (1-2): 77–83. DOI:10.1016/j.mrfmmm.2009.03.011. PMC 2807731. PMID 19428384. http://linkinghub.elsevier.com/retrieve/pii/S0027-5107(09)00115-8.
- ^ Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ (2005). "Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde". Nucleic Acids Res. 33 (11): 3513–20. DOI:10.1093/nar/gki661. PMC 1156964. PMID 15972793. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=15972793.
- ^ Friedenson B (2012). "Preventing hereditary cancers caused by opportunistic carcinogens". J Med Med Sci 3: 160–178. http://interesjournals.org/JMMS/Contents/2012%20Contents/March.htm.
- ^ Friedenson B, “Preventing hereditary cancers associated with BRCA1 and BRCA2 gene mutations” 2012
- ^ Wooster R, Weber BL (June 2003). "Breast and ovarian cancer". N. Engl. J. Med. 348 (23): 2339–47. DOI:10.1056/NEJMra012284. PMID 12788999. http://content.nejm.org/cgi/content/full/348/23/2339.
- ^ Saslow, D; Hannan, J; Osuch, J; Alciati, MH; Baines, C; Barton, M; Bobo, JK; Coleman, C et al. (2004). "Clinical breast examination: practical recommendations for optimizing performance and reporting". CA: a cancer journal for clinicians 54 (6): 327–44. DOI:10.3322/canjclin.54.6.327. PMID 15537576. edit
- ^ Yu, YH; Liang, C; Yuan, XZ (2010). "Diagnostic value of vacuum-assisted breast biopsy for breast carcinoma: a meta-analysis and systematic review.". Breast cancer research and treatment 120 (2): 469–79. DOI:10.1007/s10549-010-0750-1. PMID 20130983.
- ^ Merck Manual, Professional Edition, Ch. 253, Breast Cancer.
- ^ Sotiriou C, Pusztai L (February 2009). "Gene-expression signatures in breast cancer". N. Engl. J. Med. 360 (8): 790–800. DOI:10.1056/NEJMra0801289. PMID 19228622. http://content.nejm.org/cgi/content/full/360/8/790.
- ^ Romond EH, Perez EA, Bryant J, et al. (October 2005). "Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer". N. Engl. J. Med. 353 (16): 1673–84. DOI:10.1056/NEJMoa052122. PMID 16236738.
- ^ Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC (October 2010). "Physical activity and risk of breast cancer among postmenopausal women". Arch. Intern. Med. 170 (19): 1758–64. DOI:10.1001/archinternmed.2010.363. PMID 20975025.
- ^ Hartmann LC, Schaid DJ, Woods JE et al. (1999). "Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer". N Engl J Med 340 (2): 77–84. DOI:10.1056/NEJM199901143400201. PMID 9887158.
- ^ Meijers-Heijboer H, van Geel B, van Putten WL et al. (2001). "Breast cancer after prophylactic bilateral mastectomy in women with BRCA1 and BRCA2 mutations". N Engl J Med 345 (3): 159–164. DOI:10.1056/NEJM200107193450301. PMID 11463009.
- ^ Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective; American Institute for Cancer Research/ World Cancer Research Fund, http://www.dietandcancerreport.org
- ^ Friedenson B (2010). "A theory that explains the tissue specificity of BRCA1/2 related and other hereditary cancers". J. Med. Med. Sci. 1 (8): 372–384.
- ^ Policy and Action for Cancer Prevention, American Institute for Cancer Prevention/World Cancer Research Fund, http://www.dietandcancerreport.org
- ^ Kösters JP, Gøtzsche PC (2003). Regular self-examination or clinical examination for early detection of breast cancer. In Kösters, Jan Peter. "Cochrane Database of Systematic Reviews". Cochrane Database Syst Rev (2): CD003373. DOI:10.1002/14651858.CD003373. PMID 12804462.
- ^ Gøtzsche PC, Nielsen M (2011). "Screening for breast cancer with mammography". Cochrane Database Syst Rev (1): CD001877. DOI:10.1002/14651858.CD001877.pub4. PMID 21249649.
- ^ "Breast Cancer: Screening". United States Preventive Services Task Force. http://www.ahrq.gov/clinic/USpstf/uspsbrca.htm.
- ^ "Breast Cancer: Screening". United States Preventive Services Task Force. http://www.ahrq.gov/clinic/3rduspstf/breastCancer/brcanrr.htm#ref31.
- ^ O'Connor, M; Rhodes, D, Hruska, C (2009 Aug). "Molecular breast imaging". Expert review of anticancer therapy 9 (8): 1073–80. DOI:10.1586/era.09.75. PMC 2748346. PMID 19671027. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2748346.
- ^ Saini KS, Taylor C, Ramirez AJ, et al (2011, August). "Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries". Annals of Oncology 23 (4): 853–9. DOI:10.1093/annonc/mdr352. PMID 21821551.
- ^ "Surgery Choices for Women with Early Stage Breast Cancer". National Cancer Institute and the National Research Center for Women & Families. August 2004. http://www.stopcancerfund.org/wp/wp-content/uploads/2009/12/booklet04bc.pdf.
- ^ University of Texas M. D. Anderson Cancer Center (2009, November 4). Early-stage, HER2-positive Breast Cancer Patients At Increased Risk Of Recurrence. ScienceDaily. http://www.sciencedaily.com/releases/2009/11/091102172028.htm Retrieved February 7, 2010
- ^ "Breast Cancer: Breast Disorders: Merck Manual Professional". Merck.com. http://www.merckmanuals.com/professional/gynecology_and_obstetrics/breast_disorders/breast_cancer.html. Retrieved 2010-11-14.
- ^ Ting Bao, Michelle A Rudek (2011). "The Clinical Pharmacology of Anastrozole". European Oncology & Haematology 7 (2): 106–8. http://www.touchoncology.com/articles/clinical-pharmacology-anastrozole.
- ^ Petit T, Dufour P, Tannock I (June 2011). "A critical evaluation of the role of aromatase inhibitors as adjuvant therapy for postmenopausal women with breast cancer". Endocr. Relat. Cancer 18 (3): R79–89. DOI:10.1530/ERC-10-0162. PMID 21502311. http://erc.endocrinology-journals.org/cgi/pmidlookup?view=long&pmid=21502311.
- ^ Jahanzeb M (August 2008). "Adjuvant trastuzumab therapy for HER2-positive breast cancer". Clin. Breast Cancer 8 (4): 324–33. DOI:10.3816/CBC.2008.n.037. PMID 18757259.
- ^ "Herceptin (trastuzumab) Adjuvant HER2+ Breast Cancer Therapy Pivotal Studies and Efficacy Data". Herceptin.com. http://www.herceptin.com/hcp/adjuvant-treatment/studies-efficacy/joint-analysis.jsp. Retrieved 2010-05-08.
- ^ "Entrez Gene: ERBB2 v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=2064.
- ^ Holmes et al. (2010). "Aspirin Intake and Survival After Breast Cancer". Journal of Clinical Oncology 28 (9): 1467–72. DOI:10.1200/JCO.2009.22.7918. PMC 2849768. PMID 20159825. http://jco.ascopubs.org/cgi/content/abstract/JCO.2009.22.7918v1.
- ^ Massarut S, Baldassare G, Belleti B, Reccanello S, D'Andrea S, Ezio C, Perin T, Roncadin M, Vaidya JS (2006). "Intraoperative radiotherapy impairs breast cancer cell motility induced by surgical wound fluid". J Clin Oncol 24 (18S): 10611. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=34291.
- ^ Belletti B, Vaidya JS, D'Andrea S, et al. (March 2008). "Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding". Clin. Cancer Res. 14 (5): 1325–32. DOI:10.1158/1078-0432.CCR-07-4453. PMID 18316551. http://clincancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=18316551.
- ^ http://www.targit.org.uk/
- ^ Vaidya JS, Joseph DJ, Tobias JS, et al. (July 2010). "Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial". Lancet 376 (9735): 91–102. DOI:10.1016/S0140-6736(10)60837-9. PMID 20570343. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(10)60837-9.
- ^ Breast cancer.org Treatment Options
- ^ "Breast Cancer: Breast Disorders: Merck Manual Professional". Merck.com. http://www.merckmanuals.com/professional/gynecology_and_obstetrics/breast_disorders/breast_cancer.html. Retrieved 2010-05-08.
- ^ Elston CW, Ellis IO (November 1991). "Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up". Histopathology 19 (5): 403–10. PMID 1757079.
- ^ Peppercorn J (2009). "Breast Cancer in Women Under 40". Oncology 23 (6). http://www.cancernetwork.com/cme/article/10165/1413886.
- ^ Pritchard KI (2009). "Ovarian Suppression/Ablation in Premenopausal ER-Positive Breast Cancer Patients". Oncology 23 (1). http://www.cancernetwork.com/display/article/10165/1366719?pageNumber=1.
- ^ a b Robb C, Haley WE, Balducci L, et al. (April 2007). "Impact of breast cancer survivorship on quality of life in older women". Critical Reviews in Oncology/hematology 62 (1): 84–91. DOI:10.1016/j.critrevonc.2006.11.003. PMID 17188505.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html. Retrieved Nov. 11, 2009.
- ^ "Breast cancer: prevention and control". World Health Organization. http://www.who.int/cancer/detection/breastcancer/en/index1.html.
- ^ Stewart B. W. and Kleihues P. (Eds): World Cancer Report. IARCPress. Lyon 2003
- ^ Laurance, Jeremy (2006-09-29). "Breast cancer cases rise 80% since Seventies". The Independent (London). http://www.independent.co.uk/life-style/health-and-wellbeing/health-news/breast-cancer-cases-rise-80-since-seventies-417990.html. Retrieved 2006-10-09.
- ^ "Breast Cancer: Statistics on Incidence, Survival, and Screening". Imaginis Corporation. 2006. http://imaginis.com/breasthealth/statistics.asp. Retrieved 2006-10-09.
- ^ Breast Cancer: Breast Cancer in Young Women WebMD. Retrieved on September 9, 2009
- ^ a b c d Olson, James Stuart (2002). Bathsheba's breast: women, cancer & history. Baltimore: The Johns Hopkins University Press. pp. 9–13. ISBN 0-8018-6936-6.
- ^ "The History of Cancer". American Cancer Society. 2002-03-25. http://www.cancer.org/docroot/CRI/content/CRI_2_6x_the_history_of_cancer_72.asp?sitearea=CRI. Retrieved 2006-10-09.
- ^ a b Olson 2002, pp. 32–33
- ^ Yalom, Marilyn (1997). A history of the breast. New York: Alfred A. Knopf. p. 234. ISBN 0-679-43459-3.
- ^ a b c d Aronowitz, Robert A. (2007). Unnatural history: breast cancer and American society. Cambridge, UK: Cambridge University Press. pp. 22–24. ISBN 0-521-82249-1.
- ^ "History of Breast Cancer". Random History. 2008-02-27. http://www.randomhistory.com/1-50/029cancer.html. Retrieved 2010-05-08.
- ^ a b c d e f g Olson 2002, pp. 102–6
- ^ Olson 2002, p. 1
- ^ Marc Lacroix (2011). A Concise History of Breast Cancer. USA: Nova Science Publishers. pp. 59–68. ISBN 978-1-61122-305-7.
- ^ Olson 2002, pp. 26,28,229
- ^ Alfredo Morabia (2004). A History of Epidemiologic Methods and Concepts. Boston: Birkhauser. pp. 301–302. ISBN 3-7643-6818-7. http://books.google.com/?id=E-OZbEmPSTkC&pg=PA301. Retrieved 2007-12-31.
- ^ a b Sulik, Gayle A. (2010). Pink Ribbon Blues: How Breast Cancer Culture Undermines Women's Health. USA: Oxford University Press. pp. 200–3. ISBN 0-19-974045-3. OCLC 535493589.
- ^ Sulik 2010, pp. 37–38
- ^ Sulik 2010, p. 4
- ^ "History of Breast Cancer Advocacy > Personal Reflections > Bob Riter's Cancer Columns > Cancer Resource Center". Crcfl.net. http://www.crcfl.net/content/view/history-of-breast-cancer-advocacy.html. Retrieved 2010-05-08.
- ^ Sulik 2010, pp. 27–72
- ^ Sulik 2010, pp. 359–361
- ^ Sulik 2010, pp. 366–8
- ^ Landeman, Anne (11 June 2008). "Pinkwashing: Can Shopping Cure Breast Cancer?". Center for Media and Democracy. http://www.prwatch.org/node/7436.
- ^ Sulik 2010, pp. 365–6
- ^ Sulik 2010, pp. 372–4
- ^ Breast cancer month overshadowed by 'pinkwashing' Oct. 09 2010, Angela Mulholland, CTV.ca News
- ^ a b c d Ehrenreich, Barbara (November 2001). "Welcome to Cancerland". Harper's Magazine. http://www.barbaraehrenreich.com/cancerland.htm.
- ^ Sulik 2010, p. 57
- ^ a b Browne, Anthony (2001-10-07). "Cancer bias puts breasts first". The Guardian (London). http://www.guardian.co.uk/society/2001/oct/07/cancercare.
- ^ Arnst, Catherine (13 June 2007). "A Gender Gap in Cancer". Bloomberg Businessweek. ISSN 0007-7135. http://www.businessweek.com/technology/content/jun2007/tc20070612_953676.htm.
- ^ Olson 2002, pp. 199–200
- ^ Ave, Melanie (10 October 2006). "Tampabay: All May Not Be in the Pink". St. Petersburg Times. http://www.sptimes.com/2006/10/06/Tampabay/All_may_not_be_in_the.shtml.
- ^ a b Aschwanden, Christie (17 August 2009). "The Trouble with Mammograms". The Los Angeles Times. http://articles.latimes.com/2009/aug/17/health/he-breast-overdiagnosis17.
- ^ Grau JJ, Estapé J, Diaz-Padrón M (July 2001). "Breast cancer in Rubens paintings". Breast Cancer Res. Treat. 68 (1): 89–93. DOI:10.1023/A:1017963211998. PMID 11678312. http://www.kluweronline.com/art.pdf?issn=0167-6806&volume=68&page=89.
- ^ Espinel CH (2002). "The portrait of breast cancer and Raphael's La Fornarina". The Lancet 360 (9350): 2061–3. DOI:10.1016/S0140-6736(02)11997-0. PMID 12504417.
- ^ Braithwaite PA, Shugg D (September 1983). "Rembrandt's Bathsheba: the dark shadow of the left breast". Ann R Coll Surg Engl 65 (5): 337–8. PMC 2494383. PMID 6351705. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2494383.
- ^ Gross A (April 2004). "An epidemic of breast cancer among models of famous artists". Breast Cancer Res. Treat. 84 (3): 293. DOI:10.1023/B:BREA.0000019965.21257.49. PMID 15026627. http://www.springerlink.com/content/h2185hr70232h643/fulltext.pdf.
- ^ Bourne RG (March 2000). "Did Rembrandt's Bathsheba really have breast cancer?". Aust N Z J Surg 70 (3): 231–2. DOI:10.1046/j.1440-1622.2000.01792.x. PMID 10765910.
- ^ a b c d e f g Connie Henke Yarbro, Debra Wujcik, Barbara Holmes Gobel, ed. (2011). Cancer nursing: principles and practice (7 ed.). Jones & Bartlett Publishers. pp. 901–905. ISBN 978-1-4496-1829-2.
- ^ San Antonio Breast Cancer Symposium Abstracts, newsletters, and other reports of the meeting.
- ^ Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ (August 2009). "Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009". Ann. Oncol. 20 (8): 1319–29. DOI:10.1093/annonc/mdp322. PMC 2720818. PMID 19535820. http://annonc.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=19535820.
- ^ Lacroix, M; Leclercq G. (2004). "Relevance of breast cancer cell lines as models for breast tumours: an update". Breast Cancer Research and Treatment 83 (3): 249–289. DOI:10.1023/B:BREA.0000014042.54925.cc. PMID 14758095.
- ^ Lacroix, M; Toillon RA, Leclercq G. (2006). "p53 and breast cancer, an update". Endocrine-related cancer 13 (2): 293–325. DOI:10.1677/erc.1.01172. PMID 16728565.
- ^ a b c d Neve, RM; et al. (2006). "A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes". Cancer Cell 10 (6): 515–527. DOI:10.1016/j.ccr.2006.10.008. PMC 2730521. PMID 17157791. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2730521.
- ^ Lasfargues, EY; Ozzello L. (1958). "Cultivation of human breast carcinomas". Journal of the National Cancer Institute 21 (6): 1131–1147. PMID 13611537.
- ^ Lasfargues, EY; Coutinho WG, Redfield ES. (1978). "Isolation of two human tumor epithelial cell lines from solid breast carcinomas". Journal of the National Cancer Institute 61 (4): 967–978. PMID 212572.
- ^ Borras, M; Lacroix M, Legros N, Leclercq G. (1997). "Estrogen receptor-negative/progesterone receptor-positive Evsa-T mammary tumor cells: a model for assessing the biological property of this peculiar phenotype of breast cancers". Cancer Letters 120 (1): 23–30. DOI:10.1016/S0304-3835(97)00285-1. PMID 9570382.
- ^ Hackett, AJ; Smith HS, Springer EL, Owens RB, Nelson-Rees WA, Riggs JL, Gardner MB. (1977). "Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines". Journal of the National Cancer Institute 58 (6): 1795–1806. PMID 864756.
- ^ Soule, HD; Vazguez J, Long A, Albert S, Brennan M. (1973). "A human cell line from a pleural effusion derived from a breast carcinoma". Journal of the National Cancer Institute 51 (5): 1409–1416. PMID 4357757.
- ^ Cailleau, R; Young R, Olivé M, Reeves WJ Jr. (1974). "Breast tumor cell lines from pleural effusions". Journal of the National Cancer Institute 53 (3): 661–674. PMID 4412247.
- ^ Engel, LW; Young NA. (1978). "Human breast carcinoma cells in continuous culture: a review". Cancer Research 38 (11 Pt 2): 4327–4339. PMID 212193.
- ^ Keydar, I; Chen L, Karby S, Weiss FR, Delarea J, Radu M, Chaitcik S, Brenner HJ. (1979). "Establishment and characterization of a cell line of human breast carcinoma origin". European Journal of Cancer 15 (5): 659–670. PMID 228940.