- go to the top
- About WN
- Contact
- Feedback
- Privacy Policy
- © 2012 World News Inc., all Rights Reserved
-
Fritz London
Fritz Wolfgang London (March 7, 1900–March 30, 1954) was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces (London dispersion forces) are today considered classic and are discussed in standard textbooks of physical chemistry.
http://wn.com/Fritz_London -
Gilbert N. Lewis
Gilbert Newton Lewis (October 23, 1875 – March 23, 1946) was an American physical chemist known for the discovery of the covalent bond (see his Lewis dot structures and his 1916 paper "The Atom and the Molecule"), his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and bases, and his photochemical experiments. In 1926, Lewis coined the term "photon" for the smallest unit of radiant energy. He was a brother of Alpha Chi Sigma, the professional chemistry fraternity, and for most of his long professor career, a professor of chemistry at the University of California, Berkeley.
http://wn.com/Gilbert_N_Lewis -
Irving Langmuir

Irving Langmuir (31 January 1881 – 16 August 1957) was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his "concentric theory of atomic structure". Langmuir became embroiled in a priority dispute with Lewis over this work; Langmuir's presentation skills were largely responsible for the popularization of the theory, although the credit for the theory itself belongs mostly to Lewis. While at General Electric, from 1909–1950, Langmuir advanced several basic fields of physics and chemistry, invented the gas-filled incandescent lamp, the hydrogen welding technique, and was awarded the 1932 Nobel Prize in Chemistry for his work in surface chemistry. He was the first industrial chemist to become a Nobel laureate. The Langmuir Laboratory for Atmospheric Research near Socorro, New Mexico was named in his honor as was the American Chemical Society journal for Surface Science, called Langmuir.
http://wn.com/Irving_Langmuir -
Walter Heitler

Walter Heinrich Heitler (2 January 1904 – 15 November 1981) was a German physicist who made contributions to quantum electrodynamics and quantum field theory. He brought chemistry under quantum mechanics through his theory of valence bonding.
http://wn.com/Walter_Heitler
-
Carlton, Victoria

Carlton is an inner city suburb of Melbourne, Victoria, Australia, 2 km north from Melbourne's central business district. Its Local Government Area is the City of Melbourne. At the 2006 Census, Carlton had a population of 12,050.
http://wn.com/Carlton_Victoria
- atom
- atomic orbitals
- Bond order
- Bonding in solids
- Carlton, Victoria
- chemical bond
- Covalent radius
- delocalized electron
- diamond
- Disulfide bond
- electron
- electronegativity
- ethanol
- Fritz London
- Gilbert N. Lewis
- graphite
- Hydrogen bond
- iodine
- ionic bond
- Irving Langmuir
- Lewis structure
- Merriam-Webster
- Metallic bonding
- molecular hydrogen
- Noncovalent bonding
- pi bond
- polar covalent bond
- quantum mechanics
- quartz
- resistivity
- sigma bond
- valence (chemistry)
- valence bond theory
- Walter Heitler

- Order: Reorder
- Duration: 0:34
- Published: 13 Sep 2006
- Uploaded: 18 Feb 2012
- Author: ppornelubio

- Order: Reorder
- Duration: 4:32
- Published: 22 Oct 2009
- Uploaded: 27 Feb 2012
- Author: greatpacificmedia

- Order: Reorder
- Duration: 1:57
- Published: 15 Apr 2008
- Uploaded: 26 Feb 2012
- Author: kosasihiskandarsjah

- Order: Reorder
- Duration: 6:20
- Published: 23 Sep 2009
- Uploaded: 19 Feb 2012
- Author: MarkRosengarten

- Order: Reorder
- Duration: 2:35
- Published: 03 Sep 2010
- Uploaded: 25 Feb 2012
- Author: brightstorm2

- Order: Reorder
- Duration: 13:22
- Published: 26 Aug 2009
- Uploaded: 25 Feb 2012
- Author: khanacademy
- Order: Reorder
- Duration: 2:09
- Published: 30 Apr 2010
- Uploaded: 25 Feb 2012
- Author: TutorVista

- Order: Reorder
- Duration: 8:57
- Published: 17 Oct 2010
- Uploaded: 26 Feb 2012
- Author: bozemanbiology

- Order: Reorder
- Duration: 1:28
- Published: 15 Apr 2008
- Uploaded: 29 Jan 2012
- Author: kosasihiskandarsjah

- Order: Reorder
- Duration: 2:03
- Published: 03 Oct 2011
- Uploaded: 11 Feb 2012
- Author: DevastatingAnimation

- Order: Reorder
- Duration: 6:19
- Published: 13 Jan 2010
- Uploaded: 21 Feb 2012
- Author: MarkRosengarten

- Order: Reorder
- Duration: 7:19
- Published: 10 Nov 2009
- Uploaded: 25 Feb 2012
- Author: EducatorVids

- Order: Reorder
- Duration: 6:05
- Published: 23 Sep 2009
- Uploaded: 22 Feb 2012
- Author: MarkRosengarten

- Order: Reorder
- Duration: 8:08
- Published: 28 Aug 2010
- Uploaded: 15 Feb 2012
- Author: Learning4Mastery

- Order: Reorder
- Duration: 2:26
- Published: 03 Sep 2010
- Uploaded: 24 Feb 2012
- Author: brightstorm2


- Order: Reorder
- Duration: 1:37
- Published: 15 Apr 2008
- Uploaded: 12 Feb 2012
- Author: kosasihiskandarsjah


- Order: Reorder
- Duration: 8:06
- Published: 29 Oct 2009
- Uploaded: 04 Oct 2011
- Author: Papapodcasts

- Order: Reorder
- Duration: 9:16
- Published: 02 Oct 2008
- Uploaded: 14 Feb 2012
- Author: Papapodcasts

- Order: Reorder
- Duration: 3:10
- Published: 28 Aug 2010
- Uploaded: 21 Feb 2012
- Author: Learning4Mastery

-
 Russia's Putin warns West not to meddle
Daily Press
Russia's Putin warns West not to meddle
Daily Press
-
 Attack on Iran would be an act of criminal stupidity
Gulf News
Attack on Iran would be an act of criminal stupidity
Gulf News
-
 Taliban bomber kills nine at NATO base over Koran
Jakarta Globe
Taliban bomber kills nine at NATO base over Koran
Jakarta Globe
-
 1 killed, 4 wounded in school shooting in Ohio
Austin American Statesman
1 killed, 4 wounded in school shooting in Ohio
Austin American Statesman
-
 Taliban militants say they shot down US drone
The Star
Taliban militants say they shot down US drone
The Star
- agostic complex
- atom
- atomic orbitals
- Bond order
- Bonding in solids
- Carlton, Victoria
- chemical bond
- Covalent radius
- delocalized electron
- diamond
- Disulfide bond
- electron
- electronegativity
- ethanol
- Fritz London
- Gilbert N. Lewis
- graphite
- Hydrogen bond
- iodine
- ionic bond
- Irving Langmuir
- Lewis structure
- Merriam-Webster
- Metallic bonding
- molecular hydrogen
- Noncovalent bonding
- pi bond
- polar covalent bond
- quantum mechanics
- quartz
- resistivity
- sigma bond
- valence (chemistry)
- valence bond theory
- Walter Heitler
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Covalent bonding includes many kinds of interaction, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, and three-center two-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require the two atoms be of the same elements, only that they be of comparable electronegativity. Although covalent bonding entails sharing of electrons, it is not necessarily delocalized. Furthermore, in contrast to electrostatic interactions ("ionic bonds") the strength of covalent bond depends on the angular relation between atoms in polyatomic molecules.
History
The term "covalence" in regard to bonding was first used in 1919 by Irving Langmuir in a ''Journal of the American Chemical Society'' article entitled "The Arrangement of Electrons in Atoms and Molecules". Langmuir wrote that "we shall denote by the term covalence the number of pairs of electrons which a given atom shares with its neighbors."
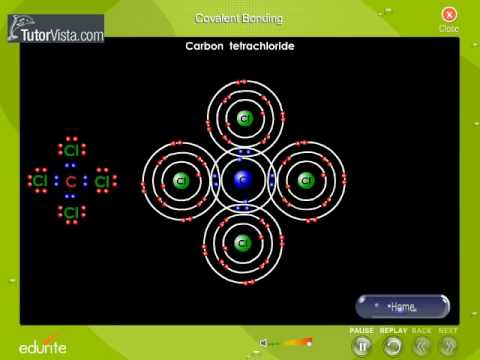
The idea of covalent bonding can be traced several years before 1919 to Gilbert N. Lewis, who in 1916 described the sharing of electron pairs between atoms. He introduced the ''Lewis notation'' or ''electron dot notation'' or ''Lewis dot structure'' in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such as double and triple bonds. Some examples of Electron Dot Notation are shown in the following figure. An alternative form of representation, not shown here, has bond-forming electron pairs represented as solid lines. While the idea of shared electron pairs provides an effective qualitative picture of covalent bonding, quantum mechanics is needed to understand the nature of these bonds and predict the structures and properties of simple molecules. Walter Heitler and Fritz London are credited with the first successful quantum mechanical explanation of a chemical bond, specifically that of molecular hydrogen, in 1927. Their work was based on the valence bond model, which assumes that a chemical bond is formed when there is good overlap between the atomic orbitals of participating atoms. These atomic orbitals are known to have specific angular relationships between each other, and thus the valence bond model can successfully predict the bond angles observed in simple molecules.
Physical properties of covalent compounds (polar/non-polar)
| Physical Properties !! Covalent Compounds | |
| States (at room temperature) | Solid, liquid, gas |
| Electrical conductivity | Usually none |
| Boiling point and Melting Point | Varies, but usually lower than ionic compounds |
| Solubility in water | Varies, but usually lower than ionic compounds |
| Thermal conductivity | Usually low |
Polarity of covalent bonds
Covalent bonds are affected by the electronegativity of the connected atoms. Two atoms with equal electronegativity will make non-polar covalent bonds such as H-H. An unequal relationship creates a polar covalent bond such as with H-Cl.
Subdivision of covalent bonds
There are three types of covalent substances: individual molecules, molecular structures, and macromolecular structures. Individual molecules have strong bonds that hold the atoms together, but there are negligible forces of attraction between molecules. Such covalent substances are gases. For example, HCl, SO2, CO2, and CH4. In molecular structures, there are weak forces of attraction. Such covalent substances are low-boiling-temperature liquids (such as ethanol), and low-melting-temperature solids (such as iodine and solid CO2). Macromolecular structures have large numbers of atoms linked in chains or sheets (such as graphite), or in 3-dimensional structures (such as diamond and quartz). These substances have high melting and boiling points, are frequently brittle, and tend to have high electrical resistivity. Elements that have high electronegativity, and the ability to form three or four electron pair bonds, often form such large macromolecular structures.
See also
References
Sources
External links
af:Kovalente binding ar:رابطة تساهمية bs:Kovalentna veza bg:Ковалентна химична връзка ca:Enllaç covalent cs:Kovalentní vazba cy:Bond cofalent da:Kovalent binding de:Atombindung et:Kovalentne side el:Ομοιοπολικός δεσμός es:Enlace covalente eu:Lotura kobalente fa:پیوند کووالانسی fr:Liaison covalente gv:Nastey co-ioosagh gl:Enlace covalente ko:공유 결합 hi:संयोजी बंध hr:Kovalentna veza id:Ikatan kovalen it:Legame covalente he:קשר קוולנטי ka:კოვალენტური ბმა kk:Коваленттік байланыс ht:Lyezon kovalan lt:Kovalentinis ryšys hu:Kovalens kötés mk:Ковалентна врска ml:സഹസംയോജകബന്ധനം mr:सहसंयुज बंध nl:Covalente binding ja:共有結合 no:Kovalent binding nn:Kovalent binding oc:Ligam covalent pl:Wiązanie kowalencyjne pt:Ligação covalente ru:Ковалентная связь si:සහසංයුජ බන්ධනය simple:Covalent bond sk:Kovalentná väzba sl:Kovalentna vez sr:Ковалентна веза su:Beungkeut kovalén fi:Kovalenttinen sidos sv:Kovalent bindning ta:சகப் பிணைப்பு th:พันธะโควาเลนต์ tr:Kovalent bağ uk:Ковалентний зв'язок vi:Liên kết cộng hóa trị zh:共价键
This text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.

