An
immune system is a
system of biological structures and
processes within an
organism that protects against
disease by identifying and killing
pathogens and
tumor cells. It detects a wide variety of agents, from
viruses to
parasitic worms, and needs to distinguish them from the organism's own healthy
cells and
tissues in order to function properly. Detection is complicated as pathogens can
evolve rapidly, and
adapt to avoid the immune system and allow the pathogens to successfully infect their
hosts.
To survive this challenge, multiple mechanisms evolved that recognize and neutralize pathogens. Even simple unicellular organisms such as bacteria possess enzyme systems that protect against viral infections. Other basic immune mechanisms evolved in ancient eukaryotes and remain in their modern descendants, such as plants and insects. These mechanisms include antimicrobial peptides called defensins, phagocytosis, and the complement system. Jawed vertebrates, including humans, have even more sophisticated defense mechanisms. The typical vertebrate immune system consists of many types of proteins, cells, organs, and tissues that interact in an elaborate and dynamic network. As part of this more complex immune response, the human immune system adapts over time to recognize specific pathogens more efficiently. This adaptation process is referred to as "adaptive immunity" or "acquired immunity" and creates immunological memory. Immunological memory, created from a primary response to a specific pathogen, provides an enhanced response to secondary encounters with that same, specific pathogen. This process of acquired immunity is the basis of vaccination. Primary response can take 2 days and up to 2 weeks to develop. After the body gains immunity towards a certain pathogen, when infection by that pathogen occurs again, the immune response is called the secondary response.
Disorders in the immune system can result in disease, including autoimmune diseases, inflammatory diseases and cancer.
Immunodeficiency diseases occur when the immune system is less active than normal, resulting in recurring and life-threatening infections. Immunodeficiency can either be the result of a genetic disease, such as severe combined immunodeficiency, or be produced by pharmaceuticals or an infection, such as the acquired immune deficiency syndrome (AIDS) that is caused by the retrovirus HIV. In contrast, autoimmune diseases result from a hyperactive immune system attacking normal tissues as if they were foreign organisms. Common autoimmune diseases include Hashimoto's thyroiditis, rheumatoid arthritis, diabetes mellitus type 1, and lupus erythematosus. Immunology covers the study of all aspects of the immune system, having significant relevance to health and diseases. Further investigation in this field is expected to play a serious role in promotion of health and treatment of diseases.
History of immunology
Immunology is a science that examines the structure and function of the immune system. It originates from
medicine and early studies on the causes of immunity to disease. The earliest known mention of immunity was during the
plague of Athens in 430 BC.
Thucydides noted that people who had recovered from a previous bout of the disease could nurse the sick without contracting the illness a second time. In the 18th century,
Pierre-Louis Moreau de Maupertuis made experiments with scorpion venom and observed that certain dogs and mice were immune to this venom. This and other observations of acquired immunity were later exploited by
Louis Pasteur in his development of
vaccination and his proposed
germ theory of disease. Pasteur's theory was in direct opposition to contemporary theories of disease, such as the
miasma theory. It was not until
Robert Koch's 1891
proofs, for which he was awarded a
Nobel Prize in 1905, that
microorganisms were confirmed as the cause of
infectious disease. Viruses were confirmed as human pathogens in 1901, with the discovery of the
yellow fever virus by
Walter Reed.
Immunology made a great advance towards the end of the 19th century, through rapid developments, in the study of humoral immunity and cellular immunity. Particularly important was the work of Paul Ehrlich, who proposed the side-chain theory to explain the specificity of the antigen-antibody reaction; his contributions to the understanding of humoral immunity were recognized by the award of a Nobel Prize in 1908, which was jointly awarded to the founder of cellular immunology, Elie Metchnikoff.
Layered defense
The immune system protects organisms from
infection with layered defenses of increasing specificity. In simple terms, physical barriers prevent pathogens such as
bacteria and
viruses from entering the organism. If a pathogen breaches these barriers, the
innate immune system provides an immediate, but non-specific response. Innate immune systems are found in all
plants and
animals. If pathogens successfully evade the innate response, vertebrates possess a second layer of protection, the
adaptive immune system, which is activated by the innate response. Here, the immune system adapts its response during an infection to improve its recognition of the pathogen. This improved response is then retained after the pathogen has been eliminated, in the form of an
immunological memory, and allows the adaptive immune system to mount faster and stronger attacks each time this pathogen is encountered.
Both innate and adaptive immunity depend on the ability of the immune system to distinguish between self and non-self molecules. In immunology, ''self'' molecules are those components of an organism's body that can be distinguished from foreign substances by the immune system. Conversely, ''non-self'' molecules are those recognized as foreign molecules. One class of non-self molecules are called antigens (short for ''anti''body ''gen''erators) and are defined as substances that bind to specific immune receptors and elicit an immune response.
Surface barriers
Several barriers protect organisms from infection, including mechanical, chemical, and biological barriers. The waxy
cuticle of many
leaves, the
exoskeleton of
insects, the
shells and membranes of externally deposited
eggs, and
skin are examples of mechanical barriers that are the first line of defense against infection. However, as organisms cannot be completely sealed against their environments, other systems act to protect body openings such as the
lungs,
intestines, and the
genitourinary tract. In the lungs,
coughing and
sneezing mechanically eject pathogens and other
irritants from the
respiratory tract. The flushing action of
tears and
urine also mechanically expels pathogens, while
mucus secreted by the respiratory and
gastrointestinal tract serves to trap and entangle
microorganisms.
Chemical barriers also protect against infection. The skin and respiratory tract secrete antimicrobial peptides such as the β-defensins. Enzymes such as lysozyme and phospholipase A2 in saliva, tears, and breast milk are also antibacterials. Vaginal secretions serve as a chemical barrier following menarche, when they become slightly acidic, while semen contains defensins and zinc to kill pathogens. In the stomach, gastric acid and proteases serve as powerful chemical defenses against ingested pathogens.
Within the genitourinary and gastrointestinal tracts, commensal flora serve as biological barriers by competing with pathogenic bacteria for food and space and, in some cases, by changing the conditions in their environment, such as pH or available iron. This reduces the probability that pathogens will be able to reach sufficient numbers to cause illness. However, since most antibiotics non-specifically target bacteria and do not affect fungi, oral antibiotics can lead to an “overgrowth” of fungi and cause conditions such as a vaginal candidiasis (a yeast infection). There is good evidence that re-introduction of probiotic flora, such as pure cultures of the lactobacilli normally found in unpasteurized yoghurt, helps restore a healthy balance of microbial populations in intestinal infections in children and encouraging preliminary data in studies on bacterial gastroenteritis, inflammatory bowel diseases, urinary tract infection and post-surgical infections.
Innate
Microorganisms or toxins that successfully enter an organism will encounter the cells and mechanisms of the innate immune system. The innate response is usually triggered when microbes are identified by
pattern recognition receptors, which recognize components that are conserved among broad groups of microorganisms, or when damaged, injured or stressed cells send out alarm signals, many of which (but not all) are recognized by the same receptors as those that recognize pathogens. Innate immune defenses are non-specific, meaning these systems respond to pathogens in a generic way. This system does not confer long-lasting
immunity against a pathogen. The innate immune system is the dominant system of host defense in most organisms.
Humoral and chemical barriers
Inflammation
Inflammation is one of the first responses of the immune system to infection. The symptoms of inflammation are redness, swelling, heat, and pain which are caused by increased
blood flow into a tissue. Inflammation is produced by
eicosanoids and
cytokines, which are released by injured or infected cells. Eicosanoids include
prostaglandins that produce
fever and the
dilation of
blood vessels associated with inflammation, and
leukotrienes that attract certain
white blood cells (leukocytes). Common cytokines include
interleukins that are responsible for communication between white blood cells;
chemokines that promote
chemotaxis; and
interferons that have
anti-viral effects, such as shutting down
protein synthesis in the host cell.
Growth factors and cytotoxic factors may also be released. These cytokines and other chemicals recruit immune cells to the site of infection and promote healing of any damaged tissue following the removal of pathogens.
Complement system
The complement system is a
biochemical cascade that attacks the surfaces of foreign cells. It contains over 20 different proteins and is named for its ability to “complement” the killing of pathogens by
antibodies. Complement is the major
humoral component of the innate immune response. Many species have complement systems, including non-
mammals like plants, fish, and some
invertebrates.
In humans, this response is activated by complement binding to antibodies that have attached to these microbes or the binding of complement proteins to carbohydrates on the surfaces of microbes. This recognition signal triggers a rapid killing response. The speed of the response is a result of signal amplification that occurs following sequential proteolytic activation of complement molecules, which are also proteases. After complement proteins initially bind to the microbe, they activate their protease activity, which in turn activates other complement proteases, and so on. This produces a catalytic cascade that amplifies the initial signal by controlled positive feedback. The cascade results in the production of peptides that attract immune cells, increase vascular permeability, and opsonize (coat) the surface of a pathogen, marking it for destruction. This deposition of complement can also kill cells directly by disrupting their plasma membrane.
Cellular barriers
Leukocytes (
white blood cells) act like independent, single-celled organisms and are the second arm of the innate immune system. The innate leukocytes include the
phagocytes (
macrophages,
neutrophils, and
dendritic cells),
mast cells,
eosinophils,
basophils, and
natural killer cells. These cells identify and eliminate pathogens, either by attacking larger pathogens through contact or by engulfing and then killing microorganisms. Innate cells are also important mediators in the activation of the
adaptive immune system.
Phagocytosis is an important feature of cellular innate immunity performed by cells called 'phagocytes' that engulf, or eat, pathogens or particles. Phagocytes generally patrol the body searching for pathogens, but can be called to specific locations by cytokines. Once a pathogen has been engulfed by a phagocyte, it becomes trapped in an intracellular vesicle called a phagosome, which subsequently fuses with another vesicle called a lysosome to form a phagolysosome. The pathogen is killed by the activity of digestive enzymes or following a respiratory burst that releases free radicals into the phagolysosome. Phagocytosis evolved as a means of acquiring nutrients, but this role was extended in phagocytes to include engulfment of pathogens as a defense mechanism. Phagocytosis probably represents the oldest form of host defense, as phagocytes have been identified in both vertebrate and invertebrate animals.
Neutrophils and macrophages are phagocytes that travel throughout the body in pursuit of invading pathogens. Neutrophils are normally found in the bloodstream and are the most abundant type of phagocyte, normally representing 50% to 60% of the total circulating leukocytes. During the acute phase of inflammation, particularly as a result of bacterial infection, neutrophils migrate toward the site of inflammation in a process called chemotaxis, and are usually the first cells to arrive at the scene of infection. Macrophages are versatile cells that reside within tissues and produce a wide array of chemicals including enzymes, complement proteins, and regulatory factors such as interleukin 1. Macrophages also act as scavengers, ridding the body of worn-out cells and other debris, and as antigen-presenting cells that activate the adaptive immune system.
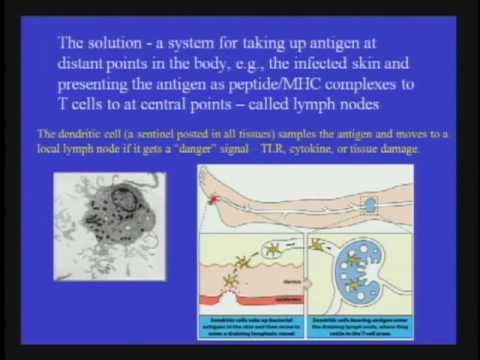
Dendritic cells (DC) are phagocytes in tissues that are in contact with the external environment; therefore, they are located mainly in the skin, nose, lungs, stomach, and intestines. They are named for their resemblance to neuronal dendrites, as both have many spine-like projections, but dendritic cells are in no way connected to the nervous system. Dendritic cells serve as a link between the bodily tissues and the innate and adaptive immune systems, as they present antigen to T cells, one of the key cell types of the adaptive immune system.
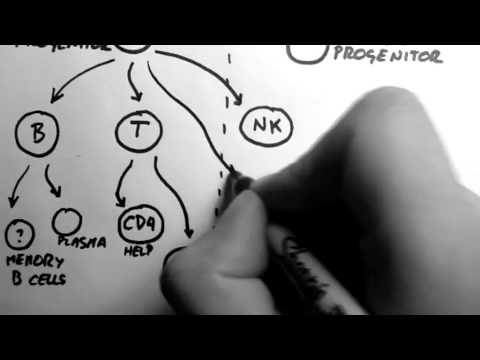
Mast cells reside in connective tissues and mucous membranes, and regulate the inflammatory response. They are most often associated with allergy and anaphylaxis. Basophils and eosinophils are related to neutrophils. They secrete chemical mediators that are involved in defending against parasites and play a role in allergic reactions, such as asthma. Natural killer (NK cells) cells are leukocytes that attack and destroy tumor cells, or cells that have been infected by viruses.
Adaptive
The adaptive immune system evolved in early vertebrates and allows for a stronger immune response as well as immunological memory, where each pathogen is "remembered" by a signature antigen. The adaptive immune response is antigen-specific and requires the recognition of specific “non-self” antigens during a process called antigen presentation. Antigen specificity allows for the generation of responses that are tailored to specific pathogens or pathogen-infected cells. The ability to mount these tailored responses is maintained in the body by "memory cells". Should a pathogen infect the body more than once, these specific memory cells are used to quickly eliminate it.
Lymphocytes
The cells of the adaptive immune system are special types of leukocytes, called
lymphocytes.
B cells and
T cells are the major types of lymphocytes and are derived from
hematopoietic stem cells in the
bone marrow. B cells are involved in the
humoral immune response, whereas T cells are involved in
cell-mediated immune response.
Both B cells and T cells carry receptor molecules that recognize specific targets. T cells recognize a “non-self” target, such as a pathogen, only after antigens (small fragments of the pathogen) have been processed and presented in combination with a “self” receptor called a major histocompatibility complex (MHC) molecule. There are two major subtypes of T cells: the killer T cell and the helper T cell. Killer T cells only recognize antigens coupled to Class I MHC molecules, while helper T cells only recognize antigens coupled to Class II MHC molecules. These two mechanisms of antigen presentation reflect the different roles of the two types of T cell. A third, minor subtype are the γδ T cells that recognize intact antigens that are not bound to MHC receptors.
In contrast, the B cell antigen-specific receptor is an antibody molecule on the B cell surface, and recognizes whole pathogens without any need for antigen processing. Each lineage of B cell expresses a different antibody, so the complete set of B cell antigen receptors represent all the antibodies that the body can manufacture.
Killer T cells
Killer T cell are a sub-group of T cells that kill cells that are infected with viruses (and other pathogens), or are otherwise damaged or dysfunctional. As with B cells, each type of T cell recognises a different antigen. Killer T cells are activated when their
T cell receptor (TCR) binds to this specific antigen in a complex with the MHC Class I receptor of another cell. Recognition of this MHC:antigen complex is aided by a
co-receptor on the T cell, called
CD8. The T cell then travels throughout the body in search of cells where the MHC I receptors bear this antigen. When an activated T cell contacts such cells, it releases
cytotoxins, such as
perforin, which form pores in the target cell's
plasma membrane, allowing
ions, water and toxins to enter. The entry of another toxin called
granulysin (a protease) induces the target cell to undergo
apoptosis. T cell killing of host cells is particularly important in preventing the replication of viruses. T cell activation is tightly controlled and generally requires a very strong MHC/antigen activation signal, or additional activation signals provided by "helper" T cells (see below).
Helper T cells
Helper T cells regulate both the innate and adaptive immune responses and help determine which types of immune responses the body will make to a particular pathogen. These cells have no cytotoxic activity and do not kill infected cells or clear pathogens directly. They instead control the immune response by directing other cells to perform these tasks.
Helper T cells express T cell receptors (TCR) that recognize antigen bound to Class II MHC molecules. The MHC:antigen complex is also recognized by the helper cell's CD4 co-receptor, which recruits molecules inside the T cell (e.g., Lck) that are responsible for the T cell's activation. Helper T cells have a weaker association with the MHC:antigen complex than observed for killer T cells, meaning many receptors (around 200–300) on the helper T cell must be bound by an MHC:antigen in order to activate the helper cell, while killer T cells can be activated by engagement of a single MHC:antigen molecule. Helper T cell activation also requires longer duration of engagement with an antigen-presenting cell. The activation of a resting helper T cell causes it to release cytokines that influence the activity of many cell types. Cytokine signals produced by helper T cells enhance the microbicidal function of macrophages and the activity of killer T cells. In addition, helper T cell activation causes an upregulation of molecules expressed on the T cell's surface, such as CD40 ligand (also called CD154), which provide extra stimulatory signals typically required to activate antibody-producing B cells.
γδ T cells
γδ T cells possess an alternative
T cell receptor (TCR) as opposed to CD4+ and CD8+ (αβ) T cells and share the characteristics of helper T cells, cytotoxic T cells and NK cells. The conditions that produce responses from γδ T cells are not fully understood. Like other 'unconventional' T cell subsets bearing invariant TCRs, such as
CD1d-restricted
Natural Killer T cells, γδ T cells straddle the border between innate and adaptive immunity. On one hand, γδ T cells are a component of
adaptive immunity as they
rearrange TCR genes to produce receptor diversity and can also develop a memory phenotype. On the other hand, the various subsets are also part of the innate immune system, as restricted TCR or NK receptors may be used as
pattern recognition receptors. For example, large numbers of human Vγ9/Vδ2 T cells respond within hours to
common molecules produced by microbes, and highly restricted Vδ1+ T cells in
epithelia will respond to stressed epithelial cells. This antigen/antibody complex is taken up by the B cell and processed by
proteolysis into peptides. The B cell then displays these antigenic peptides on its surface MHC class II molecules. This combination of MHC and antigen attracts a matching helper T cell, which releases
lymphokines and activates the B cell. As the activated B cell then begins to
divide, its offspring (
plasma cells)
secrete millions of copies of the antibody that recognizes this antigen. These antibodies circulate in blood plasma and
lymph, bind to pathogens expressing the antigen and mark them for destruction by
complement activation or for uptake and destruction by phagocytes. Antibodies can also neutralize challenges directly, by binding to bacterial toxins or by interfering with the receptors that viruses and bacteria use to infect cells.
Alternative adaptive immune system
Although the classical molecules of the adaptive immune system (e.g., antibodies and
T cell receptors) exist only in jawed vertebrates, a distinct
lymphocyte-derived molecule has been discovered in primitive
jawless vertebrates, such as the
lamprey and
hagfish. These animals possess a large array of molecules called variable lymphocyte receptors (VLRs) that, like the antigen receptors of jawed vertebrates, are produced from only a small number (one or two) of
genes. These molecules are believed to bind pathogenic
antigens in a similar way to antibodies, and with the same degree of specificity.
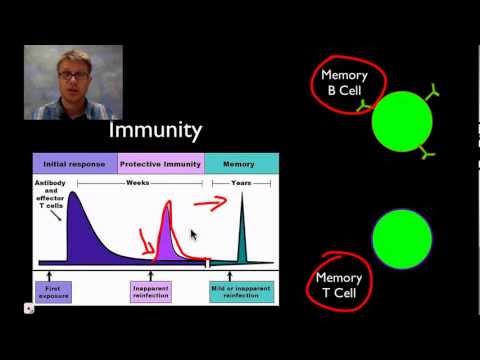
Immunological memory
When B cells and T cells are activated and begin to replicate, some of their offspring will become long-lived memory cells. Throughout the lifetime of an animal, these memory cells will remember each specific pathogen encountered and can mount a strong response if the pathogen is detected again. This is "adaptive" because it occurs during the lifetime of an individual as an adaptation to infection with that pathogen and prepares the immune system for future challenges. Immunological memory can be in the form of either passive short-term memory or active long-term memory.
Passive memory
Newborn
infants have no prior exposure to microbes and are particularly vulnerable to infection. Several layers of passive protection are provided by the mother. During
pregnancy, a particular type of antibody, called
IgG, is transported from mother to baby directly across the
placenta, so human babies have high levels of antibodies even at birth, with the same range of antigen specificities as their mother.
Breast milk or
colostrum also contains antibodies that are transferred to the gut of the infant and protect against bacterial infections until the newborn can synthesize its own antibodies. This is
passive immunity because the
fetus does not actually make any memory cells or antibodies—it only borrows them. This passive immunity is usually short-term, lasting from a few days up to several months. In medicine, protective passive immunity can also be
transferred artificially from one individual to another via antibody-rich
serum.
Active memory and immunization
Long-term ''active'' memory is acquired following infection by activation of B and T cells. Active immunity can also be generated artificially, through
vaccination. The principle behind vaccination (also called
immunization) is to introduce an
antigen from a pathogen in order to stimulate the immune system and develop
specific immunity against that particular pathogen without causing disease associated with that organism. This deliberate induction of an immune response is successful because it exploits the natural specificity of the immune system, as well as its inducibility. With infectious disease remaining one of the leading causes of death in the human population, vaccination represents the most effective manipulation of the immune system mankind has developed.
Most viral vaccines are based on live attenuated viruses, while many bacterial vaccines are based on acellular components of micro-organisms, including harmless toxin components. Since many antigens derived from acellular vaccines do not strongly induce the adaptive response, most bacterial vaccines are provided with additional adjuvants that activate the antigen-presenting cells of the innate immune system and maximize immunogenicity.
Disorders of human immunity
The immune system is a remarkably effective structure that incorporates specificity, inducibility and adaptation. Failures of host defense do occur, however, and fall into three broad categories: immunodeficiencies, autoimmunity, and hypersensitivities.
Immunodeficiencies
Immunodeficiencies occur when one or more of the components of the immune system are inactive. The ability of the immune system to respond to pathogens is diminished in both the young and the
elderly, with immune responses beginning to decline at around 50 years of age due to
immunosenescence. In
developed countries,
obesity,
alcoholism, and drug use are common causes of poor immune function. However,
malnutrition is the most common cause of immunodeficiency in
developing countries. Diets lacking sufficient protein are associated with impaired cell-mediated immunity, complement activity, phagocyte function,
IgA antibody concentrations, and cytokine production. Deficiency of single nutrients such as
iron;
copper;
zinc;
selenium;
vitamins
A,
C,
E, and
B6; and
folic acid (vitamin B
9) also reduces immune responses. Additionally, the loss of the
thymus at an early age through
genetic mutation or surgical removal results in severe immunodeficiency and a high susceptibility to infection.
Immunodeficiencies can also be inherited or 'acquired'. Chronic granulomatous disease, where phagocytes have a reduced ability to destroy pathogens, is an example of an inherited, or congenital, immunodeficiency. AIDS and some types of cancer cause acquired immunodeficiency.
Autoimmunity
Overactive immune responses comprise the other end of immune dysfunction, particularly the
autoimmune disorders. Here, the immune system fails to properly distinguish between self and non-self, and attacks part of the body. Under normal circumstances, many T cells and antibodies react with “self” peptides. One of the functions of specialized cells (located in the
thymus and
bone marrow) is to present young lymphocytes with self antigens produced throughout the body and to eliminate those cells that recognize self-antigens, preventing autoimmunity.
Hypersensitivity
Hypersensitivity is an immune response that damages the body's own tissues. They are divided into four classes (Type I – IV) based on the mechanisms involved and the time course of the hypersensitive reaction. Type I hypersensitivity is an immediate or
anaphylactic reaction, often associated with
allergy. Symptoms can range from mild discomfort to death. Type I hypersensitivity is mediated by
IgE, which triggers degranulation of
mast cells and
basophils when cross-linked by antigen.
Type II hypersensitivity occurs when antibodies bind to antigens on the patient's own cells, marking them for destruction. This is also called antibody-dependent (or cytotoxic) hypersensitivity, and is mediated by
IgG and
IgM antibodies.
Immune complexes (aggregations of antigens, complement proteins, and IgG and IgM antibodies) deposited in various tissues trigger Type III hypersensitivity reactions. Type IV hypersensitivity (also known as cell-mediated or ''delayed type hypersensitivity'') usually takes between two and three days to develop. Type IV reactions are involved in many autoimmune and infectious diseases, but may also involve ''
contact dermatitis'' (
poison ivy). These reactions are mediated by
T cells,
monocytes, and
macrophages.
Other mechanisms
It is likely that a multicomponent, adaptive immune system arose with the first
vertebrates, as
invertebrates do not generate lymphocytes or an antibody-based humoral response. Many species, however, utilize mechanisms that appear to be precursors of these aspects of vertebrate immunity. Immune systems appear even in the structurally most simple forms of life, with bacteria using a unique defense mechanism, called the
restriction modification system to protect themselves from viral pathogens, called
bacteriophages. Prokaryotes also possess acquired immunity, through a system that uses
CRISPR sequences to retain fragments of the genomes of phage that they have come into contact with in the past, which allows them to block virus replication through a form of
RNA interference.
Pattern recognition receptors are proteins used by nearly all organisms to identify molecules associated with pathogens. Antimicrobial peptides called defensins are an evolutionarily conserved component of the innate immune response found in all animals and plants, and represent the main form of invertebrate systemic immunity. The complement system and phagocytic cells are also used by most forms of invertebrate life. Ribonucleases and the RNA interference pathway are conserved across all eukaryotes, and are thought to play a role in the immune response to viruses.
Unlike animals, plants lack phagocytic cells, but many plant immune responses involve systemic chemical signals that are sent through a plant. Individual plant cells respond to molecules associated with pathogens known as Pathogen-associated molecular patterns or PAMPs. When a part of a plant becomes infected, the plant produces a localized hypersensitive response, whereby cells at the site of infection undergo rapid apoptosis to prevent the spread of the disease to other parts of the plant. Systemic acquired resistance (SAR) is a type of defensive response used by plants that renders the entire plant resistant to a particular infectious agent. RNA silencing mechanisms are particularly important in this systemic response as they can block virus replication.
Tumor immunology
Another important role of the immune system is to identify and eliminate
tumors. The ''transformed cells'' of tumors express
antigens that are not found on normal cells. To the immune system, these antigens appear foreign, and their presence causes immune cells to attack the transformed tumor cells. The antigens expressed by tumors have several sources; some are derived from
oncogenic viruses like
human papillomavirus, which causes
cervical cancer, while others are the organism's own proteins that occur at low levels in normal cells but reach high levels in tumor cells. One example is an
enzyme called
tyrosinase that, when expressed at high levels, transforms certain skin cells (e.g.
melanocytes) into tumors called
melanomas. A third possible source of tumor antigens are proteins normally important for regulating
cell growth and survival, that commonly mutate into cancer inducing molecules called
oncogenes.
The main response of the immune system to tumors is to destroy the abnormal cells using killer T cells, sometimes with the assistance of helper T cells. Tumor antigens are presented on MHC class I molecules in a similar way to viral antigens. This allows killer T cells to recognize the tumor cell as abnormal. NK cells also kill tumorous cells in a similar way, especially if the tumor cells have fewer MHC class I molecules on their surface than normal; this is a common phenomenon with tumors. Sometimes antibodies are generated against tumor cells allowing for their destruction by the complement system.
Clearly, some tumors evade the immune system and go on to become cancers. Tumor cells often have a reduced number of MHC class I molecules on their surface, thus avoiding detection by killer T cells. Some tumor cells also release products that inhibit the immune response; for example by secreting the cytokine TGF-β, which suppresses the activity of macrophages and lymphocytes. In addition, immunological tolerance may develop against tumor antigens, so the immune system no longer attacks the tumor cells.
Paradoxically, macrophages can promote tumor growth when tumor cells send out cytokines that attract macrophages, which then generate cytokines and growth factors that nurture tumor development. In addition, a combination of hypoxia in the tumor and a cytokine produced by macrophages induces tumor cells to decrease production of a protein that blocks metastasis and thereby assists spread of cancer cells.
Physiological regulation
Hormones can act as
immunomodulators, altering the sensitivity of the immune system. For example,
female sex hormones are known
immunostimulators of both adaptive and innate immune responses. Some autoimmune diseases such as
lupus erythematosus strike women preferentially, and their onset often coincides with
puberty. By contrast,
male sex hormones such as
testosterone seem to be
immunosuppressive. Other hormones appear to regulate the immune system as well, most notably
prolactin,
growth hormone and
vitamin D.
When a T-cell encounters a foreign pathogen, it extends a vitamin D receptor. This is essentially a signaling device that allows the T-cell to bind to the active form of vitamin D, the steroid hormone calcitriol. T-cells have a symbiotic relationship with vitamin D. Not only does the T-cell extend a vitamin D receptor, in essence asking to bind to the steroid hormone version of vitamin D, calcitriol, but the T-cell expresses the gene CYP27B1, which is the gene responsible for converting the pre-hormone version of vitamin D, calcidiol into the steroid hormone version, calcitriol. Only after binding to calcitriol can T-cells perform their intended function. Other immune system cells that are known to express CYP27B1 and thus activate vitamin D calcidiol, are dendritic cells, keratinocytes and macrophages.
It is conjectured that a progressive decline in hormone levels with age is partially responsible for weakened immune responses in aging individuals. Conversely, some hormones are regulated by the immune system, notably thyroid hormone activity. The age-related decline in immune function is also related to dropping vitamin D levels in the elderly. As people age, two things happen that negatively affect their vitamin D levels. First, they stay indoors more due to decreased activity levels. This means that they get less sun and therefore produce less cholecalciferol via UVB radiation. Second, as a person ages the skin becomes less adept at producing vitamin D.
The immune system is affected by sleep and rest, and sleep deprivation is detrimental to immune function. Complex feedback loops involving cytokines, such as interleukin-1 and tumor necrosis factor-α produced in response to infection, appear to also play a role in the regulation of non-rapid eye movement (REM) sleep. Thus the immune response to infection may result in changes to the sleep cycle, including an increase in slow-wave sleep relative to REM sleep.
Nutrition and diet
The functioning of the immune system, like most systems in the body, is dependent on proper nutrition. It has been long known that severe malnutrition leads to
immunodeficiency.
Overnutrition is also associated with diseases such as
diabetes and
obesity, which are known to affect immune function. More moderate malnutrition, as well as certain specific trace mineral and nutrient deficiencies, can also compromise the immune response.
Specific foods may also affect the immune system; for example, fresh fruits, vegetables, and foods rich in certain fatty acids may foster a healthy immune system. Likewise, fetal undernourishment can cause a lifelong impairment of the immune system. In traditional medicine, some herbs are believed to stimulate the immune system, such as echinacea, licorice, ginseng, astragalus, sage, garlic, elderberry, and hyssop, as well as honey although further research is needed to understand their mode of action.
Medicinal mushrooms like Shiitake, Lingzhi mushrooms, the Turkey tail mushroom, ''Agaricus blazei'', Chaga (Inonotus Obliquus) and Maitake have shown some evidence of immune system up-regulation in ''in vitro'' and ''in vivo'' studies, as well as in a limited number of clinical studies. Research suggests that the compounds in medicinal mushrooms most responsible for up-regulating the immune system are a diverse collection of polysaccharides, particularly beta-glucans, and to a lesser extent, alpha-glucans (such as Active Hexose Correlated Compound isolated from Shiitake). Alternatively, various forms of beta-glucan can be extracted from oat, barley, and yeast cell walls.
The mechanisms by which beta-glucans stimulate the immune system is only partially understood. One mechanism by which beta-glucans are thought affect immune function is through interaction with the complement receptor 3 (CD18), which is expressed on several types of immune cells. Other receptors–such as Toll-like receptor 2, Dectin-1, lactosylceramide, and scavenger receptors–have also been identified as being able to receive signals from beta-glucans.
Manipulation in medicine
The immune response can be manipulated to suppress unwanted responses resulting from autoimmunity, allergy, and
transplant rejection, and to stimulate protective responses against pathogens that largely elude the immune system (see
immunization).
Immunosuppressive drugs are used to control autoimmune disorders or
inflammation when excessive tissue damage occurs, and to prevent
transplant rejection after an
organ transplant.
Anti-inflammatory drugs are often used to control the effects of inflammation. The glucocorticoids are the most powerful of these drugs; however, these drugs can have many undesirable side effects (''e.g.'', central obesity, hyperglycemia, osteoporosis) and their use must be tightly controlled. Therefore, lower doses of anti-inflammatory drugs are often used in conjunction with cytotoxic or immunosuppressive drugs such as methotrexate or azathioprine. Cytotoxic drugs inhibit the immune response by killing dividing cells such as activated T cells. However, the killing is indiscriminate and other constantly dividing cells and their organs are affected, which causes toxic side effects. Immunosuppressive drugs such as ciclosporin prevent T cells from responding to signals correctly by inhibiting signal transduction pathways.
Larger drugs (>500 Da) can provoke a neutralizing immune response, particularly if the drugs are administered repeatedly, or in larger doses. This limits the effectiveness of drugs based on larger peptides and proteins (which are typically larger than 6000 Da). In some cases, the drug itself is not immunogenic, but may be co-administered with an immunogenic compound, as is sometimes the case for Taxol. Computational methods have been developed to predict the immunogenicity of peptides and proteins, which are particularly useful in designing therapeutic antibodies, assessing likely virulence of mutations in viral coat particles, and validation of proposed peptide-based drug treatments. Early techniques relied mainly on the observation that hydrophilic amino acids are overrepresented in epitope regions than hydrophobic amino acids; however, more recent developments rely on machine learning techniques using databases of existing known epitopes, usually on well-studied virus proteins, as a training set. A publicly accessible database has been established for the cataloguing of epitopes from pathogens known to be recognizable by B cells. The emerging field of bioinformatics-based studies of immunogenicity is referred to as ''immunoinformatics''.. Immunoproteomics is a term used to describe the study of large sets of proteins (proteomics) involved in the immune response.
Manipulation by pathogens
The success of any pathogen is dependent on its ability to elude host immune responses. Therefore, pathogens have evolved several methods that allow them to successfully infect a host, while evading detection or destruction by the immune system. Bacteria often overcome physical barriers by secreting
enzymes that digest the barrier — for example, by using a
type II secretion system. Alternatively, using a
type III secretion system, they may insert a hollow tube into the host cell, providing a direct route for proteins to move from the pathogen to the host. These proteins are often used to shut down host defenses.
An evasion strategy used by several pathogens to avoid the innate immune system is to hide within the cells of their host (also called intracellular pathogenesis). Here, a pathogen spends most of its life-cycle inside host cells, where it is shielded from direct contact with immune cells, antibodies and complement. Some examples of intracellular pathogens include viruses, the food poisoning bacterium ''Salmonella'' and the eukaryotic parasites that cause malaria (''Plasmodium falciparum'') and leishmaniasis (''Leishmania spp.''). Other bacteria, such as ''Mycobacterium tuberculosis'', live inside a protective capsule that prevents lysis by complement. Many pathogens secrete compounds that diminish or misdirect the host's immune response. Some bacteria form biofilms to protect themselves from the cells and proteins of the immune system. Such biofilms are present in many successful infections, e.g., the chronic ''Pseudomonas aeruginosa'' and ''Burkholderia cenocepacia'' infections characteristic of cystic fibrosis. Other bacteria generate surface proteins that bind to antibodies, rendering them ineffective; examples include ''Streptococcus'' (protein G), ''Staphylococcus aureus'' (protein A), and ''Peptostreptococcus magnus'' (protein L).
The mechanisms used to evade the adaptive immune system are more complicated. The simplest approach is to rapidly change non-essential epitopes (amino acids and/or sugars) on the surface of the pathogen, while keeping essential epitopes concealed. This is called antigenic variation. An example is HIV, which mutates rapidly, so the proteins on its viral envelope that are essential for entry into its host target cell are constantly changing. These frequent changes in antigens may explain the failures of vaccines directed at this virus. The parasite ''Trypanosoma brucei'' uses a similar strategy, constantly switching one type of surface protein for another, allowing it to stay one step ahead of the antibody response. Masking antigens with host molecules is another common strategy for avoiding detection by the immune system. In HIV, the envelope that covers the viron is formed from the outermost membrane of the host cell; such "self-cloaked" viruses make it difficult for the immune system to identify them as "non-self" structures.
Literature
Tzianabos, Arthur O. ''Polysaccharide Immunomodulators as Therapeutic Agents'', Harvard Medical School, Boston, USA, 2000
Cristina Lull, Harry J. Wichers, and Huub F. J. Savelkoul "Antiinflammatory and Immunomodulating Properties of Fungal Metabolites", Wageningen University and Research Center, The Netherlands 2005
See also
Clonal selection
Hapten
Human physiology
Immunoproteomics
Immunostimulator
Original antigenic sin
Tumor antigens
Immune system receptors
Polyclonal response
Plant disease resistance
Immune network theory
References
External links
Immune System - from the University of Hartford (high school/undergraduate level)
Microbiology and Immunology On-Line Textbook - from the University of South Carolina School of Medicine (undergraduate level)
Immunobiology; Fifth Edition – Online version of the textbook by Charles Janeway (Advanced undergraduate/graduate level)
iBioSeminar on Immune System - Immune System seminar by Ira Mellman
Immunology - BioMed Central a free content scientific journal
The Inner Life of a Cell - Rendering of the inner functions of the human body
The Microbial World
Cellular Immunology - Elsevier scientific journal
*
Category:Immunology
ar:جهاز مناعي
be:Імунная сістэма
be-x-old:Імунная сыстэма
bs:Imuni sistem
bg:Имунна система
ca:Sistema immunitari
cv:Иммун системи
cs:Imunitní systém
cy:System imiwnedd
da:Immunforsvar
de:Immunsystem
et:Immuunsüsteem
el:Ανοσοποιητικό σύστημα
es:Sistema inmunitario
eo:Imuna sistemo
eu:Immunitate-sistema
fa:دستگاه ایمنی
fr:Système immunitaire
gl:Sistema inmunitario
hak:Miên-yi̍t Hì-thúng
ko:면역계
hi:प्रतिरक्षा प्रणाली
hr:Imunološki sustav
id:Imunitas
it:Sistema immunitario
he:מערכת החיסון
lt:Imuninė sistema
hu:Immunrendszer
mk:Имунолошки систем
ml:രോഗപ്രതിരോധവ്യവസ്ഥ
fj:Sotia ni Yago
nl:Afweer
ne:प्रतिरक्षा प्रणाली
ja:免疫系
no:Immunforsvar
pl:Układ odpornościowy
pt:Sistema imunitário
ro:Sistemul imunitar
qu:Unquy hark'ay
ru:Иммунная система
simple:Immune system
sk:Imunitný systém
sr:Имунски систем
sh:Imunski sistem
fi:Immuunijärjestelmä
sv:Immunförsvar
tl:Sistemang imyuno
ta:நோய் எதிர்ப்பாற்றல் முறைமை
te:రోగ నిరోధక వ్యవస్థ
tr:Bağışıklık sistemi
uk:Імунна система
ur:مناعی نظام
vi:Hệ miễn dịch
yi:אימיון סיסטעם
zh:免疫系统