- Order:
- Duration: 4:23
- Published: 14 Aug 2007
- Uploaded: 02 Sep 2011
- Author: deerforum















![Let's Play Bioshock 2 - Bonus Episode (Plasmids) [1080p] Let's Play Bioshock 2 - Bonus Episode (Plasmids) [1080p]](http://web.archive.org./web/20110903134119im_/http://i.ytimg.com/vi/EQsAhKcJknA/0.jpg)












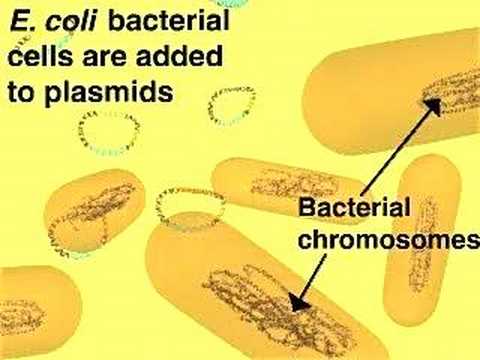
Plasmid sizes vary from 1 to over 1,000 kilobase pairs (kbp). The number of identical plasmids in a single cell can range anywhere from one to even thousands under some circumstances. Plasmids can be considered part of the mobilome because they are often associated with conjugation, a mechanism of horizontal gene transfer.
The term plasmid was first introduced by the American molecular biologist Joshua Lederberg in 1952.
Plasmids are considered transferable genetic elements, or "replicons", capable of autonomous replication within a suitable host. Plasmids can be found in all three major domains: Archea, Bacteria and Eukarya. Unlike viruses, plasmids are "naked" DNA and do not encode genes necessary to encase the genetic material for transfer to a new host, though some classes of plasmids encode the sex pilus necessary for their own transfer. Plasmid host-to-host transfer requires direct, mechanical transfer by conjugation or changes in host gene expression allowing the intentional uptake of the genetic element by transformation.
One way of grouping plasmids is by their ability to transfer to other bacteria. Conjugative plasmids contain tra genes, which perform the complex process of conjugation, the transfer of plasmids to another bacterium (Fig. 4). Non-conjugative plasmids are incapable of initiating conjugation, hence they can only be transferred with the assistance of conjugative plasmids. An intermediate class of plasmids are mobilizable, and carry only a subset of the genes required for transfer. They can parasitize a conjugative plasmid, transferring at high frequency only in its presence. Plasmids are now being used to manipulate DNA and may possibly be a tool for curing many diseases.
It is possible for plasmids of different types to coexist in a single cell. Several different plasmids have been found in E. coli. However, related plasmids are often incompatible, in the sense that only one of them survives in the cell line, due to the regulation of vital plasmid functions. Therefore, plasmids can be assigned into compatibility groups.
Another way to classify plasmids is by function. There are five main classes:
Plasmids can belong to more than one of these functional groups.
Plasmids that exist only as one or a few copies in each bacterium are, upon cell division, in danger of being lost in one of the segregating bacteria. Such single-copy plasmids have systems which attempt to actively distribute a copy to both daughter cells. These systems are often referred to as the partition system or partition function of a plasmid.
Some plasmids or microbial hosts include an addiction system or postsegregational killing system (PSK), such as the hok/sok (host killing/suppressor of killing) system of plasmid R1 in Escherichia coli. This variant produces both a long-lived poison and a short-lived antidote. Several types of plasmid addiction systems (toxin/ antitoxin, metabolism-based, ORT systems) were described in the literature and used in biotechnical (fermentation) or biomedical (vaccine therapy) applications. Daughter cells that retain a copy of the plasmid survive, while a daughter cell that fails to inherit the plasmid dies or suffers a reduced growth-rate because of the lingering poison from the parent cell. Finally, the overall productivity could be enhanced.
There are several methods to isolate plasmid DNA from bacteria, the archetypes of which are the miniprep and the maxiprep/bulkprep. The former can be used to quickly find out whether the plasmid is correct in any of several bacterial clones. The yield is a small amount of impure plasmid DNA, which is sufficient for analysis by restriction digest and for some cloning techniques.
In the latter, much larger volumes of bacterial suspension are grown from which a maxi-prep can be performed. Essentially this is a scaled-up miniprep followed by additional purification. This results in relatively large amounts (several micrograms) of very pure plasmid DNA.
In recent times many commercial kits have been created to perform plasmid extraction at various scales, purity and levels of automation. Commercial services can prepare plasmid DNA at quoted prices below $300/mg in milligram quantities and $15/mg in gram quantities ().
The rate of migration for small linear fragments is directly proportional to the voltage applied at low voltages. At higher voltages, larger fragments migrate at continually increasing yet different rates. Therefore the resolution of a gel decreases with increased voltage.
At a specified, low voltage, the migration rate of small linear DNA fragments is a function of their length. Large linear fragments (over 20kb or so) migrate at a certain fixed rate regardless of length. This is because the molecules 'resperate', with the bulk of the molecule following the leading end through the gel matrix. Restriction digests are frequently used to analyse purified plasmids. These enzymes specifically break the DNA at certain short sequences. The resulting linear fragments form 'bands' after gel electrophoresis. It is possible to purify certain fragments by cutting the bands out of the gel and dissolving the gel to release the DNA fragments.
Because of its tight conformation, supercoiled DNA migrates faster through a gel than linear or open-circular DNA.
Category:Molecular biology Category:Mobile genetic elements Category:Molecular biology techniques Category:Microbiology Category:Gene delivery
This text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.


