2:10

Dynamics of Single-stranded DNA Deamination by AID
Dynamics of Single-stranded DNA Deamination by AID
Random patterns of deamination by the enzyme activation-induced deoxycytidine deaminase (AID) are the key to generating antibody diversity, a crucial component to a healthy immune system, according to a study by researchers at the USC Dana and David Dornsife College of Letters, Arts and Sciences. Read more at bit.ly This research was originally published in The Journal of Biological Chemistry. Phuong Pham, Peter Calabrese, Soo Jung Park and Myron F. Goodman. An analysis of a single-stranded DNA scanning process in which AID deaminates C to U haphazardly and inefficiently to ensure mutational diversity. The Journal of Biological Chemistry. 2011; Vol. 286: 24931--24942. © the American Society for Biochemistry and Molecular Biology.
7:16

Metabolism - 1/3
Metabolism - 1/3
part one of three LICENSE: Creative Commons (Attribution-Noncommercial-Share Alike) The copyright owner allows distribution and also creation of derivative works of this video, in each case with attribution and under the same or similar license as this license, but prohibits commercial use. For more information about this license, please read: creativecommons.org
1:16

AMP Deaminase and the Purine Nucleotide Cycle in HD.mp4
AMP Deaminase and the Purine Nucleotide Cycle in HD.mp4
The purine nucleotide cycle involves conversion of aspartate to fumarate, a key TCA cycle intermediate. During exercise, muscles utilize the purine nucleotide cycle to upregulate TCA intermediates. AMP deaminase is key in generating IMP via a deamination reaction. IMP is necessary for subsequent reconversion to AMP via an addition of aspartate to form adenylosuccinate. Loss of fumarate from adenylosuccinate both restores AMP and is the step at which purines can be used to generate TCA intermediates for muscle. A congenital deficiency in this enzyme can yield cramping and fatigue during demanding physical activity.
0:44

MD simulation of glucosamine-6-phosphate deaminase part III
MD simulation of glucosamine-6-phosphate deaminase part III
A 10 ns molecular dynamics simulation of glucosamine-6-phosphate deaminase. A close-up of the allosteric ligand an the waters molecules that are near to it. Due to limitations of VMD I can only select the nearby water molecules at just a fixed point in time. In part III I'll show you the nearby water molecules at the begining of the simulation.
48:54
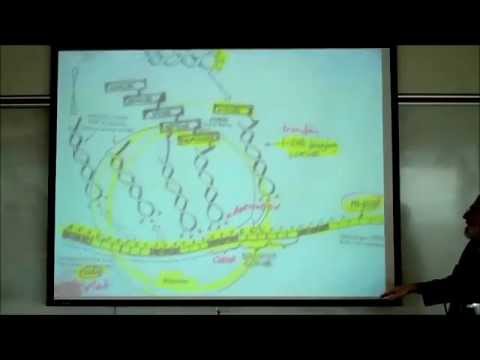
PHYSIOLOGY; TRANSCRIPTION, TRANSLATION & THE FORMATION OF UREA & URIC ACID by Professor Fink
PHYSIOLOGY; TRANSCRIPTION, TRANSLATION & THE FORMATION OF UREA & URIC ACID by Professor Fink
Review of Transcription, Translation & the Formation of Urea & Uric Acid. The Lecture includes reference to the Gene locus (segment or exon), sense strand, RNA Polymerase, messenger (m-) RNA, ribosome, transfer (t-) RNA, codon (triplet), anticodon, peptide bonds, polypeptide chain, Genetic Mutations, Genetic Diseases The Lecture also reviews the metabolism (anabolism & catabolism) of Proteins and Nucleic Acids, including: deamination, Liver, urea, Nucleic Acids, nucleotides, uric acid, BUN, hyperuricemia, gouty Arthritis (gout).
4:42

EVOLUTION MISSING GENETIC CODE
EVOLUTION MISSING GENETIC CODE
The inosine (orange colored = 6) family is shown with its fellow purine nucleotides (adenosine family = red = 1; guanosine family = green colored = 2i; pyrmidine nucleotides = 3 (Thymine family = (yellow) =4;Uracil family =5 = purple, inosine family = orange = 6. New six nucleotide genetic pairings (A+T),(U+I),(C+G) . tRNA editing Adenosine to Inosine or adenine to hypoxanthine or xanthine Wobble Code position 34 on amino acid peptide chain, and methyl inosine position 37 plus GU Wobble Metabolic Switches, Cytosine to Uracil ( C to U) deamination of pyrmidine nucleotides parallel and at same time deamination of A to I for purines; Instruction set = synthesis de novo, catabolic degradation, synthesis salvage ie wobble switches stop protein production when too much nitrogen is in the neuromuscular system and CNS glutamate removes NH3 (ammonia) from CNS; NH3 toxic, fatal to CNS and immune systems
0:44

DNA repair: Base excision repair LONG patch 1.0 - Full HD
DNA repair: Base excision repair LONG patch 1.0 - Full HD
Base excision repair (BER) pathway, protects both nuclear and mitochondrial DNA from "spontaneous DNA damage", mainly generated by eactive oxigen spices (ROS) produced by the normal metabolism of the cell. The spectrum of nucleotide base lesions includes: spontaneous or enzyme-induced deamination, oxidation or alkylation. The starrings of the BER pathway are the glycosylases enzymes. They are the DNA-damage sensors and have evolved to selectively detect different kinds of DNA insults. An important trait of the glycosylases consists in their substrate redundance, as demonstrated by mouse models lacking one glycosylase, wich triggers mild consecuences. The glycosylases can be classified as monofunctional, when, only posses the N-glycosylase function, so exclusively removes the damaged nitrogenated base of the nucleotide. When additionally has an AP-lyase activity to remove the deoxyribophosphate (dRP), generating an abasic (AP) site, they are considered as bifunctional glycosylases. MONOFUNCTIONAL DNA Glycosylases: - MPG - UNG-1 - UNG2 - SMUG - MBD4 - TDG - MYH BIFUNCTIONAL DNA Glycosylases: - OGG1 - NTH - NEIL-1 - NEIL-2 Main steps of BER long patch: 1- A depurinized, oxydized or alkylated nucleotide base is generated spontaneously or by metabolic-generated reactive oxygen species. 2- A DNA glysosylase specifically detects the damaged nitrogenated base of the nucleotide and removes it. If the damaged DNA consist in a single-strand break (SSB), the Poly (ADP-ribose <b>...</b>
42:49

PHYSIOLOGY; CELLULAR RESPIRATION; PART 2 by Professor Fink
PHYSIOLOGY; CELLULAR RESPIRATION; PART 2 by Professor Fink
Review of the use of fats and proteins in Cellular Respiration. Reference is made to deamination of amino acids, urea, glycogen, ketoacids (ketone bodies, including acetone), and gluconeogenesis..Also covered are fasting, Atkins Diet, low-carbohydrate diets, Diabetes, hyperglycemia, glycosuria, ketonemia, ketosis, ketonuria and ketoacidosis.
0:29

DNA repair : Base excision repair SHORT patch v 1.0 - Full HD
DNA repair : Base excision repair SHORT patch v 1.0 - Full HD
DNA repair : Base excision repair short patch - Full HD Base excision repair (BER) pathway, protects both nuclear and mitochondrial DNA from "spontaneous DNA damage", mainly generated by eactive oxigen spices (ROS) produced by the normal metabolism of the cell. The spectrum of nucleotide base lesions includes: spontaneous or enzyme-induced deamination, oxidation or alkylation. The starrings of the BER pathway are the glycosylases enzymes. They are the DNA-damage sensors and have evolved to selectively detect different kinds of DNA insults. An important trait of the glycosylases consists in their substrate redundance, as demonstrated by mouse models lacking one glycosylase, wich triggers mild consecuences. The glycosylases can be classified as monofunctional, when, only posses the N-glycosylase function, so exclusively removes the damaged nitrogenated base of the nucleotide. When additionally has an AP-lyase activity to remove the deoxyribophosphate (dRP), generating an abasic (AP) site, they are considered as bifunctional glycosylases. MONOFUNCTIONAL DNA Glycosylases: - MPG - UNG-1 - UNG2 - SMUG - MBD4 - TDG - MYH BIFUNCTIONAL DNA Glycosylases: - OGG1 - NTH - NEIL-1 - NEIL-2 Main steps of BER short pathway 1- A depurinized, oxydized or alkylated nucleotide base is generated spontaneously or by metabolic-generated reactive oxygen species.. 2- A DNA glysosylase specifically detects the damaged nitrogenated base of the nucleotide and removes it. 3- The XRCC1 protein, brings <b>...</b>
1:21

Biochemic
Biochemic
..... Well here's something different =.=;; Got the idea from a friend of mine. Based on the popular song "Technologic" XD ...to be honest i have no idea what half of those things are LoL ....don't ask =_=;;; Just...don't =_=;; A bit too much science =_=;; ------------------------------------------------- PIP2 PIP3 Ion channel Protein Kinase Adaptor protein GPCR Map Kinase Action potentials Phospholipase C G proteins Ligands binding Receptor proteins Calcium 2+ cAMP GAP GGL Domains Cell Signaling Cell signaling Acylation Alkylation Amidation Formylation Methylation pegylation Prenylation Farnesylation Oxidation Plus reduction Deamination Phosphorylation Flavin attachment diSulfide bridges ADP - Ribosylation Modifications Modifications Leucine Glycine Isoleucine Valine Alanine methionine Serine cysteine Asparagine Proline Threonine and glutamine Lysine Argnine Histidine Aspartate Glutamate Tyrosine Phenylalanine Tryptophan Selenocysteine Ornithine Amino acids Amino acids L-pyrrolysine Carnitine willardiine trigonelline Canavanine homoserine Mimosine furanomycin Saccharopine Indospicine Citrulline Homocitrulline Guvacine Homoarginine Lathyrine hydroxyleucine Amino Acids Amino Acids
2:13

LET A THOUSAND PROTEINS BLOOM_ABISHEK HAZRA
LET A THOUSAND PROTEINS BLOOM_ABISHEK HAZRA
VISCERAL: THE LIVING ART EXPERIMENT Science Gallery 28 January 25 February 2011 Imagine a not so distant future where rogue nation states are harnessing human biomaterials to create explosives. This work attempts to produce ammonium nitrate from breast milk. Using a process called deamination to extract ammonia from breast milk, the work interrogates popular perceptions around 'good' and 'bad' material. Staged as a failed experiment, the work draws attention to the constraining logic of utility that frames scientific research. Milk donated by The Human Milk Bank, Irvinestown, Co Fermanagh. www.sciencegallery.com/visceral
50:56

DNA Replication, Recombination, Repair III
DNA Replication, Recombination, Repair III
This course is part of a series taught by Kevin Ahern at Oregon State University on General Biochemistry. For more information about online courses go to ecampus.oregonstate.edu for the rest of the courses see www.youtube.com 1. Initiation of replication in E. coli occurs at a specific site on the E. coli genomic DNA, known as OriC, in the cell's circular chromosome. The OriC site contains three repeats of an AT rich sequence near some sequences bound by the DNA A protein. 2. Replication initiation begins with binding of the several copies of the DNA A protein to the OriC site. Bending and wrapping of the DNA around DNA A proteins causes the AT-rich sequences noted above to become single-stranded. 3. Next, the DNA BC complex binds the DNA B protein (helicase) to each of the single strands in opposite orientations. The DNA C protein is released in the process. Next, SSB and primase bind the exposed single-stranded regions and cause DNA A protein to be released. The primases begin synthesizing RNA primers (remember - 5' to 3' RNA synthesis only also) in opposite directions on each strand. The primases DO NOT require a pre-existing primer to function. 4. Note that replication is bi-directional - two replication forks pointed in opposite directions from the origin. They meet later at a termination site on the other side of the genomic DNA. 5. Eukaryotic DNA replication is coordinated tightly with the cell cycle. Checkpoints during the cell cycle ensure that progression <b>...</b>
4:32

Protein - 4/4
Protein - 4/4
part four of four LICENSE: Creative Commons (Attribution-Noncommercial-Share Alike) The copyright owner allows distribution and also creation of derivative works of this video, in each case with attribution and under the same or similar license as this license, but prohibits commercial use. For more information about this license, please read: creativecommons.org
2:12

D and A
D and A
D and A DNA I twist and turn, and I don't really mind, mind I look to replicate but things happen Damages keep me from this game we play, play Now lets fix this Mutation and damages keep distracting me, me I fix my problems really really fast I hope these damages dont actually last, last Cuz DNA The problem is that we have to know how to make this right cause if we dont cell suicide The problem is that we have to know how to make this right (right, right, right, right) DNA Lets hope polymerase finds its binding site If I dont repair Ill have to keep this change Lets hope polymerase finds its binding site Fix the zone Depurination and Deamination Is when bases are chemically attacked Within the new strand repairs are made, made Almost complete Mismatch repair protein Binds to the new strand The newly synthesized strand is removed, moved DNA then DNA polymerase repairs the gap and ligase comes to finish the job The problem is solved, we made it right, right, right, right DNA DNA polymerase found its binding site Now we dont have to keep this change The problem is solved, we made it right, right, right, right
48:24

Bite-Sized Biochemistry #43 - DNA Replication, Repair, Recombination III
Bite-Sized Biochemistry #43 - DNA Replication, Repair, Recombination III
(02/14/11) Lecture by Kevin Ahern of Oregon State University discussing Biochemistry Basics in BB 451. See the full course at oregonstate.edu This course can be taken for credit (wherever you live) via OSU's ecampus. For details, see ecampus.oregonstate.edu Download Metabolic Melodies at www.davincipress.com Related courses include BB 350 - oregonstate.edu BB 450 - oregonstate.edu BB 100 - oregonstate.edu DNA Replication/Repair/Recombination III 1. Initiation of replication in E. coli occurs at a specific site on the E. coli genomic DNA, known as OriC, in the cell's circular chromosome. The OriC site contains three repeats of an AT rich sequence near some sequences bound by the DNA A protein. 2. Replication initiation begins with binding of the several copies of the DNA A protein to the OriC site. Bending and wrapping of the DNA around DNA A proteins causes the AT-rich sequences noted above to become single-stranded. 3. Next, the DNA BC complex binds the DNA B protein (helicase) to each of the single strands in opposite orientations. The DNA C protein is released in the process (I said this backward in class). Next, SSB and primase bind the exposed single-stranded regions and cause DNA A protein to be released. The primases begin synthesizing RNA primers (remember - 5' to 3' RNA synthesis only also) in opposite directions on each strand. The primases DO NOT require a pre-existing primer to function. 4. Note that replication is bi-directional - two replication forks <b>...</b>
48:21

Bite-Sized Biochemistry #40 - Nucleotide Metabolism II
Bite-Sized Biochemistry #40 - Nucleotide Metabolism II
(02/07/11) Lecture by Kevin Ahern of Oregon State University discussing Biochemistry Basics in BB 451. See the full course at oregonstate.edu This course can be taken for credit (wherever you live) via OSU's ecampus. For details, see ecampus.oregonstate.edu Download Metabolic Melodies at www.davincipress.com Related courses include BB 350 - oregonstate.edu BB 450 - oregonstate.edu BB 100 - oregonstate.edu Highlights - Nucleotide Metabolism II 1. Ribonucleotide reductase (RNR) catalyzes the formation of deoxyribonucleotides from ribonucleotides. The substrates are ribonucleoside diphosphates (ADP, GDP, CDP, or UDP) and the products are deoxyribonucleoside diphosphates (dADP, dGDP, dCDP, or dUDP). 2. RNR has two pairs of two identical subunits - R1 (large subunit) and R2 (small subunit). R1 has two allosteric binding sites and the active site of the enzyme. R2 forms a tyrosine radical necessary for the reaction mechanism of the enzyme. 3. Ribonucleotide reductase is allosterically regulated via two binding sites - a specificity () binding site (controls which substrates the enzyme binds and which deoxyribonucleotides are made) and an activity binding site (controls whether or not enzyme is active - ATP activates, dATP inactivates). Specificity sites act in a generally complementary fashion. Binding of deoxypyrimidine triphosphates to the specificity site tends to inhibit binding and reduction of pyrimidine diphosphates at the enzyme's active site and stimulates binding and <b>...</b>
9:58

Metabolism - 2/3
Metabolism - 2/3
part two of three LICENSE: Creative Commons (Attribution-Noncommercial-Share Alike) The copyright owner allows distribution and also creation of derivative works of this video, in each case with attribution and under the same or similar license as this license, but prohibits commercial use. For more information about this license, please read: creativecommons.org
50:33

Lec 12 | MIT 7.014 Introductory Biology, Spring 2005
Lec 12 | MIT 7.014 Introductory Biology, Spring 2005
Molecular Biology III (Prof. Graham Walker) View the complete course: ocw.mit.edu License: Creative Commons BY-NC-SA More information at ocw.mit.edu More courses at ocw.mit.edu
46:05

#43 Biochemistry DNA Replication III Lecture for BB 451/551 Winter 2012
#43 Biochemistry DNA Replication III Lecture for BB 451/551 Winter 2012
A lecture by Kevin Ahern of Oregon State University to his BB 451/551 class. See the full course at oregonstate.edu This course can be taken for credit (wherever you live) via OSU's ecampus. For details, see ecampus.oregonstate.edu Topics covered include DNA replication, DNA polymerase, telomerase, RNA primers, chromosomes, chromosome ends, replication of linear ends, aging, cancer, stem cells, DNA repair, mutS, mutH, mutL, proofreading, excinuclease, excision repair, U in DNA, base excision, nucleotide excision, mismatch repair, cell cycle, telomere, 8-oxoguanine, aflatoxin, thymine dimers, psoralen, suntanning, UV radiation
34:02

Citric Acid Cycle I
Citric Acid Cycle I
This course is part of a series taught by Kevin Ahern at Oregon State University on General Biochemistry. For more information about online courses go to ecampus.oregonstate.edu for the rest of the courses see www.youtube.com The following is a summary of my lecture. I provide it (and subsequent ones) for your information and not as a mechanism of dumping more information on you. Use them if they help you to recall the material. Otherwise, don't bother. 1. Both oxidative decarboxylation (in higher cells) and non-oxidative decarboxylation (in yeast) use an enzymatic activity called the pyryvate dehydrogenase complex to convert pyruvate from glycolysis into acetyl-Coa for the citric acid cycle. This enzyme complex is in the mitochondrion and requires that pyruvate from the cytoplasm be transported to the mitochondrion. This complex includes the following: Pyruvate decarboxylase (your book calls it "Pyruvate Dehydrogenase Component" (E1) Dihyrolipoamide transacetylase (E2) Dihyrolipoamide dehydrogenase (E3) It also uses the coenzymes, Thiamine Pyrophosphate (TPP), Lipoamide, NAD, FAD, and Coenzyme A (also called CoASH or CoA). 2. The mechanism of the reaction catalyzed by the complex is very similar to that catalyzed by the alpha-keto-glutarate dehydrogenase complex of the Citric Acid Cycle. Both involve oxidation of alpha-keto acids. 3. In aerobic higher organisms, the reaction mechanism involves binding of pyruvate by an ionized TPP, decarboxylation, transfer to the <b>...</b>





