- go to the top
- About WN
- Contact
- Feedback
- Privacy Policy
- © 2012 World News Inc., all Rights Reserved
-
Australia

{{Infobox country
http://wn.com/Australia -
Cleveland Clinic

The Cleveland Clinic (formally known as the Cleveland Clinic Foundation) is a multispecialty academic medical center located in Cleveland, Ohio, United States. The Cleveland Clinic is currently regarded as one of the top 4 hospitals in the United States. The Cleveland Clinic was established in 1921 by four physicians for the purpose of providing patient care, research, and medical education in an ideal medical setting. One of the largest private medical centers in the world, the Cleveland Clinic saw more than 2,800,000 patient visits in 2005, with almost 70,000 hospital admissions. Patients arrive at the Cleveland Clinic from all 50 states and more than 100 nations. The Cleveland Clinic's approximately 1,700 salaried staff physicians represent 120 medical specialties and subspecialties. The Cleveland Clinic was ranked number one in America for cardiac care from 1994 to 2009.
http://wn.com/Cleveland_Clinic
- 5-HT receptor
- 5-HT1 receptor
- 5-HT1B receptor
- 5-HT2 receptor
- 5-HT2A receptor
- 5-HT2B receptor
- 5-HT2C receptor
- 5-HT3
- 5-HT3 antagonist
- 5-HTTLPR
- 5-hydroxytryptophan
- 5HT2C receptor
- 5HT3 receptor
- adrenaline
- agonist
- Alan Frazer
- Alpha (biology)
- amine
- amine oxide
- amino acid
- aminorex
- ammonia
- amphetamine
- Anadenanthera
- antidepressant
- antiemetic
- antipsychotic
- antitussive
- anxiolytic
- arthropod
- Australia
- banana
- beta cell
- bilateria
- Biological tissue
- Biosynthesis
- blood plasma
- blood sugar
- blood-brain barrier
- brainstem
- cabergoline
- cancer
- carcinoid
- carcinoid syndrome
- cardiac fibrosis
- cardiac myocyte
- cerebellum
- chemotherapy
- chlorphentermine
- circadian rhythm
- Cleveland Clinic
- CMAJ
- cocaine
- crayfish
- cuticle
- dauer larva
- deathstalker
- Debye
- decarboxylation
- dendrite
- dextromethorphan
- dietary supplement
- dimethyltryptamine
- dopamine
- drugs
- empathogen
- endogenous
- endothelium
- enzyme
- ergotamine
- exocytosis
- fenfluramine
- fluoxetine
- frontal cortex
- fruits
- fungi
- gastrointestinal
- granisetron
- growth factor
- GTPase
- Gut (zoology)
- hallucinogen
- heart failure
- hemostasis
- hickory
- hippocampus
- hydroxyl
- hydroxylation
- IC50
- incentive salience
- indole
- Inositol
- insulin
- insulin resistance
- intracellular
- ion channel
- Irvine Page
- Julie G. Hensler
- kiwifruit
- ligand
- lisuride
- lobster
- LSD
- MAO-B
- MAOI
- MDMA
- medulla oblongata
- mescaline
- mesencephalon
- Mesolimbic pathway
- metabolic pathway
- metabolite
- methylation
- methysergide
- migraine
- monoamine oxidase
- Mood (psychology)
- mushroom
- myenteric plexus
- nausea
- neuron
- neurons
- norepinephrine
- nucleus accumbens
- obesity
- octopamine
- ondansetron
- osteoblast
- osteoclast
- overdose
- p11 protein
- pancreas
- pergolide
- pineal gland
- pineapple
- plant
- plantain
- platelet
- platelets
- plum
- polyol
- pons
- presynaptic terminal
- Protein isoform
- psilocin
- psilocybin
- psychedelic drug
- purebred
- pyridoxal phosphate
- quorum sensing
- Radiation therapy
- raphe nuclei
- reticular formation
- reuptake
- Science (journal)
- Scientific American
- second messenger
- seeds
- serotonergic
- serotonin receptor
- serotonin syndrome
- sertraline
- spinal cord
- SSRI
- stinging nettle
- striatum
- substansia nigra
- swarm behavior
- thrombocyte
- tianeptine
- tomato
- TPH1
- TPH2
- tropisetron
- tryptamine
- tryptophan
- tumor
- type 2 diabetes
- tyramine
- ultimatum game
- urine
- vegetable
- ventromedial nucleus
- vertebrate
- Vittorio Erspamer
- vomiting
- walnut
- wasp
- weaning
- yopo

- Order: Reorder
- Duration: 3:30
- Published: 08 Mar 2011
- Uploaded: 01 Jan 2012
- Author: RoughTradeRecordsUK

- Order: Reorder
- Duration: 3:44
- Published: 15 Jun 2010
- Uploaded: 02 Jan 2012
- Author: MysteryJets

- Order: Reorder
- Duration: 4:57
- Published: 30 May 2009
- Uploaded: 29 Dec 2011
- Author: MedicineBoys

- Order: Reorder
- Duration: 1:22
- Published: 12 Nov 2009
- Uploaded: 13 Dec 2011
- Author: bluebellyvideos

- Order: Reorder
- Duration: 2:04
- Published: 31 Oct 2009
- Uploaded: 02 Jan 2012
- Author: ehowhealth

- Order: Reorder
- Duration: 7:36
- Published: 02 Jan 2007
- Uploaded: 03 Dec 2011
- Author: psychmedz1
![Nine Horses - Serotonin [Track No.8] Nine Horses - Serotonin [Track No.8]](http://web.archive.org./web/20120118072943im_/http://i.ytimg.com/vi/fofrkYwVSXQ/0.jpg)
- Order: Reorder
- Duration: 5:52
- Published: 05 Nov 2009
- Uploaded: 22 Dec 2011
- Author: visockasvaidotas

- Order: Reorder
- Duration: 3:18
- Published: 02 Jan 2011
- Uploaded: 01 Jan 2012
- Author: manbitesdogrecords
- Order: Reorder
- Duration: 5:48
- Published: 09 Feb 2010
- Uploaded: 02 Jan 2012
- Author: joshrubineastwest

- Order: Reorder
- Duration: 3:45
- Published: 23 Jan 2011
- Uploaded: 01 Jan 2012
- Author: MysteryJets

- Order: Reorder
- Duration: 3:38
- Published: 25 Feb 2009
- Uploaded: 10 Dec 2011
- Author: widescreenmode

- Order: Reorder
- Duration: 5:58
- Published: 04 Dec 2006
- Uploaded: 01 Jan 2012
- Author: simplekidstuff

- Order: Reorder
- Duration: 10:12
- Published: 22 Apr 2010
- Uploaded: 02 Jan 2012
- Author: PaulFromStokeUK


- Order: Reorder
- Duration: 3:30
- Published: 09 Mar 2011
- Uploaded: 02 Jan 2012
- Author: MysteryJets



- Order: Reorder
- Duration: 3:27
- Published: 03 Oct 2010
- Uploaded: 01 Jan 2012
- Author: SurfsUpStudios

- Order: Reorder
- Duration: 4:29
- Published: 28 Jun 2010
- Uploaded: 02 Jan 2012
- Author: MysteryJets
- Order: Reorder
- Duration: 2:40
- Published: 05 Feb 2010
- Uploaded: 26 Dec 2011
- Author: NatureVideoChannel

- Order: Reorder
- Duration: 3:09
- Published: 06 Oct 2010
- Uploaded: 02 Jan 2012
- Author: MysteryJets


- Order: Reorder
- Duration: 4:48
- Published: 06 Nov 2008
- Uploaded: 01 Jan 2012
- Author: UCtelevision
size: 10.2Kb
size: 9.2Kb
-
 Divers blast rescue hole in Italian cruise ship
CNN
Divers blast rescue hole in Italian cruise ship
CNN
-
 Costa Concordia : des témoignages accablent le commandant
Liberation - France
Costa Concordia : des témoignages accablent le commandant
Liberation - France
-
 Iran warns Gulf Arabs not to boost oil output
Richmond Times Dispatch
Iran warns Gulf Arabs not to boost oil output
Richmond Times Dispatch
-
 Caskets of Violence and State, Be Not Proud
WorldNews.com
Caskets of Violence and State, Be Not Proud
WorldNews.com
-
 Captain held as cruise ship hits rock and sinks
The Independent
Captain held as cruise ship hits rock and sinks
The Independent
- 5-HIAA
- 5-HT receptor
- 5-HT1 receptor
- 5-HT1B receptor
- 5-HT2 receptor
- 5-HT2A receptor
- 5-HT2B receptor
- 5-HT2C receptor
- 5-HT3
- 5-HT3 antagonist
- 5-HTTLPR
- 5-hydroxytryptophan
- 5HT2C receptor
- 5HT3 receptor
- adrenaline
- agonist
- Alan Frazer
- Alpha (biology)
- amine
- amine oxide
- amino acid
- aminorex
- ammonia
- amphetamine
- Anadenanthera
- antidepressant
- antiemetic
- antipsychotic
- antitussive
- anxiolytic
- arthropod
- Australia
- banana
- beta cell
- bilateria
- Biological tissue
- Biosynthesis
- blood plasma
- blood sugar
- blood-brain barrier
- brainstem
- cabergoline
- cancer
- carcinoid
- carcinoid syndrome
- cardiac fibrosis
- cardiac myocyte
- cerebellum
- chemotherapy
- chlorphentermine
- circadian rhythm
- Cleveland Clinic
- CMAJ
- cocaine
- crayfish
- cuticle
- dauer larva
- deathstalker
- Debye
- decarboxylation
size: 6.2Kb
size: 1.0Kb
size: 11.7Kb
size: 4.3Kb
size: 1.0Kb
size: 4.1Kb
size: 7.8Kb
size: 12.0Kb
size: 3.1Kb
Approximately 80 percent of the human body's total serotonin is located in the enterochromaffin cells in the gut, where it is used to regulate intestinal movements. The remainder is synthesized in serotonergic neurons in the CNS where it has various functions. These include the regulation of mood, appetite, and sleep. Serotonin also has some cognitive functions, including in memory and learning. Modulation of serotonin at synapses is thought to be a major action of several classes of pharmacological antidepressants.
Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by blood platelets, which store it. When the platelets bind to a clot, they disgorge serotonin, where it serves as a vasoconstrictor and helps to regulate hemostasis and blood clotting. Serotonin also is a growth factor for some types of cells, which may give it a role in wound healing.
Serotonin is mainly metabolized to 5-HIAA, chiefly by the liver. Metabolism involves first oxidation by monoamine oxidase ( MAO ) to the corresponding aldehyde. This is followed by oxidation by aldehyde dehydrogenase to 5-HIAA, the indole acetic acid derivative. The latter is then excreted by the kidneys. One type of tumor, called carcinoid, sometimes secretes large amounts of serotonin into the blood, which causes various forms of the carcinoid syndrome of flushing, diarrhea, and heart problems. Because of serotonin's growth promoting effect on cardiac myocytes, persons with serotonin-secreting carcinoid may suffer a right heart (tricuspid) valve disease syndrome, caused by proliferation of myocytes onto the valve.
In addition to animals, serotonin is also found in fungi and plants. Serotonin's presence in insect venoms and plant spines serves to cause pain, which is a side effect of serotonin injection. Serotonin is produced by pathogenic amoebas, and its effect on the gut causes diarrhea. Its widespread presence in many seeds and fruits may serve to stimulate the digestive tract into expelling the seeds.
Functions
Serotonin is a neurotransmitter, and is found in all bilateral animals, where it mediates gut movements and the animal's perception of resource availability. In the simplest animals, resources are equivalent with food, but in advanced animals such as arthropods and vertebrates, resources also can mean social dominance. In response to the perceived abundance or scarcity of resources, the animal's growth, reproduction or mood may be elevated or lowered. Recent studies involving the serotonin transporter gene 5-HTT have shown the short allele of this gene increases synaptic serotonin levels. These genetic studies have demonstrated serotonin has strong associations with depression in regards to a negative environment.
Gauge of food availability
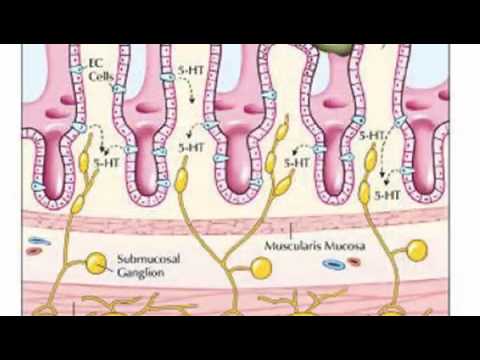
Serotonin functions as a neurotransmitter in the nervous systems of simple, as well as complex, animals. For example, in the roundworm C. elegans, which feeds on bacteria, serotonin is released as a signal in response to positive events, e.g., finding a new source of food or in male animals finding a female with which to mate. When a well-fed worm feels bacteria on its cuticle, dopamine is released, which slows it down; if it is starved, serotonin also is released, which slows the animal down further. This mechanism increases the amount of time animals spend in the presence of food. The released serotonin activates the muscles used for feeding, while octopamine suppresses them. Serotonin diffuses to serotonin-sensitive neurons, which control the animal's perception of nutrient availability. This system has been partially conserved during the 700 million years of evolution which separate C. elegans from humans. When humans smell food, dopamine is released to increase the appetite. But unlike in worms, serotonin does not increase anticipatory behaviour in humans; instead, the serotonin released while consuming activates 5-HT2C receptors on dopamine-producing cells. This halts their dopamine release, and thereby serotonin decreases appetite. Drugs which block 5-HT2C receptors make the body unable to shut off appetite, and are associated with increased weight gain, especially in people who have a low number of receptors. The expression of 5-HT2C receptors in the hippocampus follows a diurnal rhythm, just as the serotonin release in the ventromedial nucleus, which is characterised by a peak at morning when the motivation to eat is strongest.
Effects of food content
In humans, serotonin levels are affected by diet. An increase in the ratio of tryptophan to phenylalanine and leucine will increase serotonin levels. Fruits with a good ratio include dates, papayas and bananas. Foods with a lower ratio inhibit the production of serotonin. These include whole wheat and rye bread. Research also suggests eating a diet rich in carbohydrates and low in protein will increase serotonin by secreting insulin, which helps in amino acid competition. However, increasing insulin for a long period may trigger the onset of insulin resistance, obesity, type 2 diabetes, and lower serotonin levels. Muscles use many of the amino acids except tryptophan, allowing men to have more serotonin than women. Myo-inositol, a carbocyclic polyol present in many foods, is known to play a role in serotonin modulation.
In the digestive tract
The gut is surrounded by enterochromaffin cells, which release serotonin in response to food in the lumen. This makes the gut contract around the food. Platelets in the veins draining the gut collect excess serotonin.If irritants are present in the food, the enterochromaffin cells release more serotonin to make the gut move faster, i.e., to cause diarrhea, so that the gut is emptied of the noxious substance. If serotonin is released in the blood faster than the platelets can absorb it, the level of free serotonin in the blood is increased. This activates 5HT3 receptors in the chemoreceptor trigger zone that stimulate vomiting. The enterochromaffin cells not only react to bad food, they are also very sensitive to irradiation and cancer chemotherapy. Drugs that block 5HT3 are very effective in controlling the nausea and vomiting produced by cancer treatment, and are considered the gold standard for this purpose.
Gauge of social situation
How much food an animal gets not only depends on the abundance of food, but also on the animal's ability to compete with others. This is especially true for social animals, where the stronger individuals might steal food from the weaker. Thus, serotonin is not only involved in the perception of food availability, but also of social rank. If a lobster is injected with serotonin, it behaves like a dominant animal, while octopamine causes subordinate behavior. A frightened crayfish flips its tail to flee, and the effect of serotonin on this behavior depends on the animal's social status. Serotonin inhibits the fleeing reaction in subordinates, but enhances it in socially dominant or isolated individuals. The reason for this is social experience alters the proportion between serotonin receptors (5-HT receptors) that have opposing effects on the fight-or-flight response. The effect of 5-HT1 receptors predominates in subordinate animals, while 5-HT2 receptors predominates in dominants. In humans, levels of 5-HT1A receptor activation in the brain show negative correlation with aggression, and a mutation in the gene that codes for the 5-HT2A receptor may double the risk of suicide for those with that genotype. Most of the brain serotonin is not degraded after use, but is collected by serotonergic neurons by serotonin transporters on their cell surfaces. Studies have revealed nearly 10% of total variance in anxiety-related personality depends on variations in the description of where, when and how many serotonin transporters the neurons should deploy, and the effect of this variation was found to interact with the environment in depression.
Effects on growth and reproduction
In C. elegans, artificial depletion of serotonin or increase of octopamine cues behavior typical of a low-food environment: C. elegans becomes more active, and mating and egg-laying is suppressed, while the opposite occurs if serotonin is increased or octopamine is decreased in this animal. Serotonin is necessary for normal male mating behavior, and the inclination to leave food to search for a mate. The serotonergic signaling used to adapt the worm's behaviour to fast changes in the environment affects insulin-like signaling and the TGF beta signaling pathway, which control long-term adaption.
Bone metabolism
In mice and humans, alterations in serotonin levels and signalling have been shown to regulate bone mass. Mice that lack brain serotonin have osteopenia while mice that lack gut serotonin have high bone density. In humans increased blood serotonin levels have been shown to be significant negative predictor of low bone density. Serotonin can also be synthesized, albeit at a very low levels, in the bone cells. Serotonin mediates its actions on bone cells using three different receptors. Through Htr1b receptor it negatively regulates bone mass while it does so positively through Htr2b and Htr2c. These studies have opened up a new area of research in bone metabolism that can be potentially harnessed to treat bone mass disorders.
Behavior
Serotonin is necessary for normal male mating behavior, and the inclination to leave food to search for a mate. The serotonergic signaling used to adapt the worm's behaviour to fast changes in the environment affects insulin-like signaling and the TGF beta signaling pathway, which control long-term adaption. In the fruitfly, where insulin both regulates blood sugar and acts as a growth factor, serotonergic neurons regulate the adult body size by affecting insulin secretion. Serotonin has also been identified as the trigger for swarm behavior in locusts. In humans, though insulin regulates blood sugar and IGF regulates growth, serotonin controls the release of both hormones so that serotonin suppresses insulin release from the beta cells in the pancreas, and exposure to SSRIs reduces fetal growth. Human serotonin can also act as a growth factor directly. Liver damage increases cellular expression of 5-HT2A and 5-HT2B receptors. Serotonin present in the blood then stimulates cellular growth to repair liver damage. 5HT2B receptors also activate osteoblasts, which build up bone However, serotonin also activates osteoclasts, which degrade bone.In March 2011, Chinese scientists found male mice lose their heterosexuality if bred without serotonin. By taking away the tryptophan hydroxylase 2 gene, which is needed to produce serotonin, male mice were deprived of it. They also injected mice with a chemical that depletes serotonin. In that case, the male mice showed a preference for other male mice. When injected with a compound to restore serotonin, they returned to female mating preferences. However, experts have warned against making links between serotonin and human sexuality, as sexual preference in mice is largely driven by odours; humans are far less affected by odour cues.
Cardiovascular growth factor
Serotonin, in addition, evokes endothelial nitric oxide synthase activation and stimulates, through a 5-HT1B receptor-meditated mechanism, the phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures. In blood, serotonin is collected from plasma by platelets, which store it. It is thus active wherever platelets bind in damaged tissue, as a vasoconstrictor to stop bleeding, and also as a fibrocyte mitotic (growth factor), to aid healing.Some serotonergic agonist drugs also cause fibrosis anywhere in the body, particularly the syndrome of retroperitoneal fibrosis, as well as cardiac valve fibrosis. In the past, three groups of serotonergic drugs have been epidemiologically linked with these syndromes. They are the serotonergic vasoconstrictive antimigraine drugs (ergotamine and methysergide), the serotonergic appetite suppressant drugs (fenfluramine, chlorphentermine, and aminorex), and certain anti-Parkinsonian dopaminergic agonists, which also stimulate serotonergic 5-HT2B receptors. These include pergolide and cabergoline, but not the more dopamine-specific lisuride. As with fenfluramine, some of these drugs have been withdrawn from the market after groups taking them showed a statistical increase of one or more of the side effects described. An example is pergolide. The drug was declining in use since reported in 2003 to be associated with cardiac fibrosis. Two independent studies published in the New England Journal of Medicine in January 2007, implicated pergolide, along with cabergoline, in causing valvular heart disease. As a result of this, the FDA removed pergolide from the U.S. market in March, 2007. (Since cabergoline is not approved in the U.S. for Parkinson's Disease, but for hyperprolactinemia, the drug remains on the market. Treatment for hyperprolactinemia requires lower doses than that for Parkinson's Disease, diminishing the risk of valvular heart disease).
Local effects of injection: venoms and pain
Since serotonin is an indicator of bleeding, a sudden large increase in peripheral levels causes pain. The reason for wasps and deathstalkers to have serotonin in their venom may be to increase the pain of their sting on large animals, and also to cause lethal vasoconstriction in smaller prey.
Deficiency
Genetically altered C. elegans that lack serotonin have an increased reproductive lifespan, may become obese, and sometimes present with arrested development at a dormant larval state.Serotonin in mammals is made by two different tryptophan hydroxylases: TPH1 produces serotonin in the pineal gland and the enterochromaffin cells, while TPH2 produces it in the raphe nuclei and in the myenteric plexus. Genetically altered mice lacking TPH1 develop progressive loss of heart strength early on. They have pale skin and breathing difficulties, are easily tired, and eventually die of heart failure. Genetically altered mice that lack TPH2 are normal when they are born. However, after three days they appear to be smaller and weaker, and have softer skin than their siblings. In a purebred strain, 50% of the mutants died during the first four weeks, but in a mixed strain, 90% survived. Normally, the mother weans the litter after three weeks, but the mutant animals needed five weeks. After that, they caught up in growth and had normal mortality rates. Subtle changes in the autonomic nervous system are present, but the most obvious difference from normal mice is the increased aggressiveness and impairment in maternal care of young. Despite the blood-brain barrier, the loss of serotonin production in the brain is partially compensated by intestinal serotonin. The behavioural changes become greatly enhanced if one crosses TPH1- with TPH2-lacking mice and gets animals that lack TPH entirely.
In humans, defective signaling of serotonin in the brain may be the root cause of sudden infant death syndrome (SIDS). Scientists from the European Molecular Biology Laboratory in Monterotondo, Italy genetically modified lab mice to produce low levels of the neurotransmitter serotonin. The results showed the mice suffered drops in heart rate and other symptoms of SIDS, and many of the animals died at an early age. Researchers now believe low levels of serotonin in the animals' brainstems, which control heartbeat and breathing, may have caused sudden death, they said in the July 4, 2008 issue of Science. If neurons that make serotonin — serotonergic neurons — are abnormal in infants, there is a risk of sudden infant death syndrome (SIDS).
Low levels of serotonin may also be associated with intense spiritual experiences.
Recent research conducted at Rockefeller University shows, in both patients who suffer from depression and mice that model the disorder, levels of the p11 protein are decreased. This protein is related to serotonin transmission within the brain.
Anatomy
Gross anatomy
The neurons of the raphe nuclei are the principal source of 5-HT release in the brain. The raphe nuclei are neurons grouped into about nine pairs and distributed along the entire length of the brainstem, centered around the reticular formation. Axons from the neurons of the raphe nuclei form a neurotransmitter system, reaching almost every part of the central nervous system. Axons of neurons in the lower raphe nuclei terminate in the cerebellum and spinal cord, while the axons of the higher nuclei spread out in the entire brain.
Microanatomy
Serotonin is released into the space between neurons, and diffuses over a relatively wide gap (>20 µm) to activate 5-HT receptors located on the dendrites, cell bodies and presynaptic terminals of adjacent neurons.
Receptors
The 5-HT receptors are the receptors for serotonin. They are located on the cell membrane of nerve cells and other cell types in animals, and mediate the effects of serotonin as the endogenous ligand and of a broad range of pharmaceutical and hallucinogenic drugs. With the exception of the 5-HT3 receptor, a ligand-gated ion channel, all other 5-HT receptors are G protein-coupled, seven transmembrane (or heptahelical) receptors that activate an intracellular second messenger cascade.
Termination
Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT, SERT, on the presynaptic neuron. Various agents can inhibit 5-HT reuptake, including MDMA (ecstasy), amphetamine, cocaine, dextromethorphan (an antitussive), tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs). Interestingly, a 2006 study conducted by the University of Washington suggested a newly discovered monoamine transporter, known as PMAT, may account for "a significant percentage of 5-HT clearance". Contrasting with the high-affinity SERT, the PMAT has been identified as a low-affinity transporter, with an apparent Km of 114 micromoles/L for serotonin; approximately 230 times higher than that of SERT. However, the PMAT, despite its relatively low serotonergic affinity, has a considerably higher transport capacity than SERT, "..resulting in roughly comparable uptake efficiencies to SERT in heterologous expression systems." The study also suggests some SSRIs, such as fluoxetine and sertraline, inhibit PMAT but at IC50 values which surpass the therapeutic plasma concentrations by up to four orders of magnitude; therefore, SSRI monotherapy is ineffective in PMAT inhibition. At present, there are no known pharmaceuticals which would appreciably inhibit PMAT at normal therapeutic doses. The PMAT also suggestively transports dopamine and norepinephrine, albeit at Km values even higher than that of 5-HT (330–15,000 μmoles/L).
Serotonylation
Serotonin can also signal through a nonreceptor mechanism called serotonylation, in which serotonin modifies proteins. This process underlies serotonin effects upon platelet-forming cells (thrombocytes) in which it links to the modification of signaling enzymes called GTPases that then trigger the release of vesicle contents by exocytosis. A similar process underlies the pancreatic release of insulin. The effects of serotonin upon vascular smooth muscle "tone" (this is the biological function from which serotonin originally got its name) depend upon the serotonylation of proteins involved in the contractile apparatus of muscle cells.
Biosynthesis
In animals including humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC). The TPH-mediated reaction is the rate-limiting step in the pathway. TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a brain-specific isoform.Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system, because it does not cross the blood-brain barrier. However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, can and does cross the blood-brain barrier. These agents are available as dietary supplements, and may be effective serotonergic agents. One product of serotonin breakdown is 5-hydroxyindoleacetic acid (5 HIAA), which is excreted in the urine. Serotonin and 5 HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Drugs targeting the 5-HT system
Several classes of drugs target the 5-HT system, including some antidepressants, antipsychotics, anxiolytics, antiemetics, and antimigraine drugs, as well as the psychedelic drugs and empathogens.
Psychedelic drugs
The psychedelic drugs psilocin/psilocybin, DMT, mescaline, and LSD are agonists, primarily at 5HT2A/2C receptors. The empathogen-entactogen MDMA (ecstasy) releases serotonin from synaptic vesicles of neurons.
Antidepressants
The most prescribed drugs in many parts of the world are drugs which alter serotonin levels. They are used in depression, generalized anxiety disorder and social phobia. Monoamine oxidase inhibitors (MAOI) prevent the breakdown of monoamine neurotransmitters (including serotonin), and therefore increase concentrations of the neurotransmitter in the brain. MAOI therapy is associated with many adverse drug reactions, and patients are at risk of hypertensive emergency triggered by foods with high tyramine content and certain drugs. Some drugs inhibit the reuptake of serotonin, making it stay in the synapse longer. The tricyclic antidepressants (TCAs) inhibit the reuptake of both serotonin and norepinephrine. The newer selective serotonin reuptake inhibitors (SSRIs) have fewer side effects and fewer interactions with other drugs. The side effects that have become apparent recently include a decrease in bone mass in elderly and increased risk for osteoporosis. However, it is not yet clear whether it is due to SSRI action on peripheral serotonin production and or action in the gut or in the brain. Certain SSRI medications have been shown to lower serotonin levels below the baseline after chronic use, despite initial increases in serotonin. This has been connected to the observation that the benefit of SSRIs may decrease in selected patients after a long-term treatment. A switch in medication will usually resolve this issue (up to 70% of the time). The novel antidepressant tianeptine, a selective serotonin reuptake enhancer, has mood-elevating effects. This provides evidence for the theory that serotonin is most likely used to regulate the extent or intensity of moods, rather than level directly correlating with mood.Although phobias and depression might be attenuated by serotonin-altering drugs, this does not mean the individual's situation has been improved, but only the individual's perception of the environment. Sometimes, a lower serotonin level might be beneficial, for example in the ultimatum game, where players with normal serotonin levels are more prone to accept unfair offers than participants whose serotonin levels have been artificially lowered.
Serotonin syndrome
Extremely high levels of serotonin can cause a condition known as serotonin syndrome, with toxic and potentially fatal effects. In practice, such toxic levels are essentially impossible to reach through an overdose of a single antidepressant drug, but require a combination of serotonergic agents, such as an SSRI with an MAOI. The intensity of the symptoms of serotonin syndrome vary over a wide spectrum, and the milder forms are seen even at nontoxic levels.
Antiemetics
Some 5-HT3 antagonists, such as ondansetron, granisetron, and tropisetron, are important antiemetic agents. They are particularly important in treating the nausea and vomiting that occur during anticancer chemotherapy using cytotoxic drugs. Another application is in the treatment of postoperative nausea and vomiting.
In unicellular organisms
Serotonin is used by a variety of single-cell organisms for various purposes. Selective serotonin reuptake inhibitors (SSRIs) have been found to be toxic to algae. The gastrointestinal parasite Entamoeba histolytica secretes serotonin, causing a sustained secretory diarrhea in some patients. Patients infected with E. histolytica have been found to have highly elevated serum serotonin levels which returned to normal following resolution of the infection. E. histolytica also responds to the presence of serotonin by becoming more virulent. This means serotonin secretion not only serves to increase the spread of enteamoebas by giving the host diarrhea, but also to coordinate their behaviour according to their population density, a phenomenon known as quorum sensing. Outside a host, the density of entoamoebas is low, and hence also the serotonin concentration. Low serotonin signals to the entoamoebas they are outside a host and they become less virulent in order to conserve energy. When they enter a new host, they multiply in the gut, and become more virulent as the serotonin concentration increases.
In plants
In drying seeds serotonin production is a way to get rid of the buildup of poisonous ammonia. The ammonia is collected and placed in the indole part of L-tryptophan, which is then decarboxylated by tryptophan decarboxylase to give tryptamine, which is then hydroxylated by a cytochrome P450 monooxygenase, yielding serotonin.However, since serotonin is a major gastrointestinal tract modulator, it may be produced by plants in fruits as a way of speeding the passage of seeds through the digestive tract, in the same way as many well known-seed and fruit associated laxatives. Serotonin is found in mushrooms, fruits and vegetables. The highest values of 25–400 mg/kg have been found in nuts of the walnut (Juglans) and hickory (Carya) genera. Serotonin concentrations of 3–30 mg/kg have been found in plantain, pineapple, banana, kiwifruit, plums, and tomatoes. Moderate levels from 0.1–3 mg/kg have been found in a wide range of tested vegetables.
Serotonin is one compound of the poison contained in stinging nettles (Urtica dioica), where it causes pain on injection in the same manner as its presence in insect venoms (see above).
Unlike its precursors, 5-HTP and tryptophan, serotonin does not cross the blood–brain barrier, which means that ingesting serotonin in the diet has no effect on brain serotonin levels.
Methyl-tryptamines and hallucinogens
Several plants contain serotonin together with a family of related tryptamines that are methylated at the amino (NH2) and (OH) groups, are N-oxides, or miss the OH group. These compounds do reach the brain, although some portion of them are metabolized by MAO-B enzymes in the liver. Examples are plants from the Anadenanthera genus that are used in the hallucinogenic yopo snuff. These compounds are widely present in the leaves of many plants, and may serve as deterrents for animal ingestion.
History
In 1935, Italian Vittorio Erspamer showed that an extract from enterochromaffin cells made intestines contract. Some believed it contained adrenaline, but two years later Erspamer was able to show that it was a previously unknown amine, which he named enteramine. In 1948, Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic discovered a vasoconstrictor substance in blood serum, and since it was a serum agent affecting vascular tone, they named it serotonin. In 1952 it was shown that enteramine was the same substance as serotonin, and as the broad range of physiological roles were elucidated, the abbreviation 5HT of the proper chemical name 5-hydroxytryptamine became the preferred name in the pharmacological field.
References
External links
Category:Serotonin Category:Natural tryptamine alkaloids Category:Biogenic amines
ar:سيروتونين bg:Серотонин ca:Serotonina cs:Serotonin da:Serotonin de:Serotonin et:Serotoniin el:Σεροτονίνη es:Serotonina eo:Serotonino eu:Serotonina fa:سروتونین fr:Sérotonine ko:세로토닌 hy:Սերոտոնին hr:Serotonin id:Serotonin it:Serotonina he:סרוטונין kk:Серотонин la:Serotoninum lt:Serotoninas hu:Szerotonin nl:Serotonine ja:セロトニン no:Serotonin oc:Serotonina pl:Serotonina pt:Serotonina ro:Serotonină ru:Серотонин simple:Serotonin sl:Serotonin sr:Серотонин sh:Serotonin fi:Serotoniini sv:Serotonin th:เซโรโทนิน tr:Serotonin uk:Серотонін ur:Serotonin zh:血清張力素This text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.
| Coordinates | 28°36′36″N77°13′48″N |
|---|---|
| name | Simple Kid |
| background | solo_singer |
| birth name | Kieran McFeely |
| origin | Cork, Ireland |
| instrument | Vocals, guitar, banjo, harmonica |
| genre | AlternativeElectronicaFolkIndustrial rock |
| occupation | Singer-songwriter, musician |
| label | 2m, Vector Recordings, Gentlemen Recordings, Yep Roc Records |
| associated acts | The Young Offenders |
| website | SimpleKid.com |
| notable instruments | }} |
Simple Kid, real-name Ciaran McFeely, is an Irish-born solo musical artist.
History
Simple Kid's approach to recording involves recording to an 8-track cassette player then fed into his computer where he applies more modern techniques to create finished songs. He mixed and mastered the tracks on albums "1" and "2" on his own equipment. His style of remixing and sampling has been compared to Fatboy Slim's, although Simple Kid uses his own recordings as sources. More often his sound is compared to the similarly eclectic and deliberately ramshackle Beck.Simple Kid's influences include folk, country (he prominently features banjos and slide guitar in his work), glam rock (particularly T. Rex) and late 60s big pop arrangements of records like The Beatles's 'White Album'.
Before becoming Simple Kid, McFeely was a member of The Young Offenders. Forming when he was 17, the band was composed of friends made while growing up in Cork Ireland. The group attempted to release an album in America but met with failure and broke up.
Although having spent considerable time in America before becoming Simple Kid, his popularity today is mostly limited to the United Kingdom and Ireland. His touring is based out of London and he has played support for R.E.M. and Kings Of Leon,Snow patrol at ward park. among others.
Television appearances include Later... with Jools Holland, Late Night with Conan O'Brien, The Late Late Show and Other Voices on RTE. He's also been the subject of a Channel 4 (4 Play) short documentary and appeared on TFI Friday with The Young Offenders.
His second album, 2, was released by Country Gentleman Recordings in October 2006 in the UK, and by Yep Roc Records in August 2007 in the US. "2" was included in Mojo Magazine's top 50 albums of the year, was CD of the week in The Times Culture section and had positive reviews in the NME, The Guardian, Time Out and the Independent on Sunday, to name a few. The Rolling Stone Magazine also featured Simple Kid as a breakthrough act for 2007.
The track "Lil' King Kong" off "2" is featured in a Saturn automobile advertisement in the US, as well as in an advertisement in the UK for the mobile phone operator Orange. "Lil' King Kong also featured in Hollywood movie Jumper in 2008.
In early 2011 it was announced on the official website that there will be no further music or tours by Simple Kid.
Discography
1 (a.k.a. Simple Kid 1 or SK1)
Released September 2003 (UK), June 2004 (USA)
2 (a.k.a. Simple Kid 2 or SK2)
Released October 2006 (UK), August 2007 (USA)
References
External links
Category:Irish country musicians Category:Music from Cork Category:Living people Category:Year of birth missing (living people)
de:Simple Kid pl:Simple KidThis text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.
Paul Collier, CBE is a Professor of Economics, Director for the Centre for the Study of African Economies at The University of Oxford and Fellow of St Antony's College. From 1998 – 2003 he was the director of the Development Research Group of the World Bank.
Life
Collier is a specialist in the political, economical and developmental predicaments of poor countries. He was brought up in Sheffield where he attended King Edward VII School. He holds a Distinction Award from Oxford University, and in 1988 he was awarded the Edgar Graham Book Prize for the co-written Labour and poverty in rural Tanzania: Ujamaa and rural development in the United Republic of Tanzania.The Bottom Billion: Why the Poorest Countries are Failing and What Can Be Done About It (ISBN 0195311450), has been compared to Jeffrey Sachs's The End of Poverty and William Easterly's The White Man's Burden, two influential books, which like Collier's book, discuss the pros and cons of developmental aid to developing countries.
His 2010 book The Plundered Planet is encapsulated in his formulas: Nature - Technology + Regulation = Starvation, Nature + Technology - Regulation = Plunder, and Nature + Technology + Regulation (Good governance) = Prosperity. The book describes itself as an attempt at a middle way between the extremism of "Ostriches" (Denialism, particularly climate change denial) and "Environmental Romanticism" (for example, anti-genetically modified organisms movements in Europe). The book is about sustainable management in relation with the geo-politics of global warming, with an attempt to avoid a global tragedy of the commons, with prime example of overfishing. In it he builds upon a legacy of the economic psychology of greed and fear, from early Utilitarianism (Jeremy Bentham) to more recently the Stern Review.
He was appointed Commander of the Order of the British Empire (CBE) in the 2008 Birthday Honours. He is a patron of the Media Legal Defence Initiative.
In 2010, he was named by Foreign Policy magazine to its list of top global thinkers.
He is currently working for the Copenhagen Consensus, where he is the expert on conflict.
Work
Research Topics
Selected Publications
Video
Press
See also
References
External links
Category:British economists Category:Year of birth missing (living people) Category:Living people Category:Sustainability advocates Category:Development specialists Category:Climate economists Category:Commanders of the Order of the British Empire Category:Fellows of St Antony's College, Oxford Category:People educated at King Edward VII School, Sheffield
de:Paul Collier nl:Paul Collier ja:ポール・コリアー no:Paul Collier sk:Paul Collier ta:போல் கொலியர்This text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.


