
- Order:
- Duration: 7:41
- Published: 20 Mar 2008
- Uploaded: 02 Aug 2011
- Author: rastusgentibus





















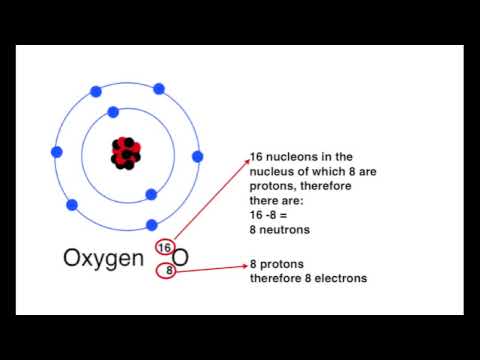













































The neutron has been the key to nuclear power production. After the neutron was discovered in 1932, it was realized in 1933 that it might mediate a nuclear chain reaction. In the 1930s, neutrons were used to produce many different types of nuclear transmutations. When nuclear fission was discovered in 1938, it was soon realized that this might be the mechanism to produce the neutrons for the chain reaction, if the process also produced neutrons, and this was proven in 1939, making the path to nuclear power production evident. These events and findings led directly to the first man-made nuclear chain reaction which was self-sustaining (Chicago Pile-1, 1942) and to the first nuclear weapons (1945).
In 1931, Walther Bothe and Herbert Becker in Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris. They showed that if this unknown radiation fell on paraffin, or any other hydrogen-containing compound, it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis.
In 1932, James Chadwick performed a series of experiments at the University of Cambridge, showing that the gamma ray hypothesis was untenable. He suggested that the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion. These uncharged particles were called neutrons, apparently from the Latin root for neutral and the Greek ending -on (by imitation of electron and proton).
The discovery of the neutron explained a puzzle involving the spin of the nitrogen-14 nucleus, which had been experimentally measured to be 1 ħ. It was known that atomic nuclei usually had about half as many positive charges as if they were composed completely of protons, and in existing models this was often explained by proposing that nuclei also contained some "nuclear electrons" to neutralize the excess charge. Thus, nitrogen-14 would be composed of 14 protons and 7 electrons to give it a charge of +7 but a mass of 14 atomic mass units. However, it was also known that both protons and electrons carried an intrinsic spin of ħ, and there was no way to arrange an odd number (21) of spins ± ħ to give a spin of 1 ħ. Instead, when nitrogen-14 was proposed to consist of 3 pairs of protons and neutrons, with an additional unpaired neutron and proton each contributing a spin of ħ in the same direction for a total spin of 1 ħ, the model became viable. Soon, nuclear neutrons were used to naturally explain spin differences in many different nuclides in the same way, and the neutron as a basic structural unit of atomic nuclei was accepted.
Under the Standard Model of particle physics, because the neutron consists of three quarks, the only possible decay mode without a change of baryon number is for one of the quarks to change flavour via the weak interaction. The neutron consists of two down quarks with charge − e and one up quark with charge + e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a W boson. By this means the neutron decays into a proton (which contains one down and two up quarks), an electron, and an electron antineutrino.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of (about 14 minutes, 46 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of ) is (about 10 minutes, 14 seconds). : → + +
Neutrons in unstable nuclei can also decay in this manner. However, inside a nucleus, protons can also transform into a neutron via inverse beta decay. This transformation occurs by emission of a antielectron (also called positron) and a neutrino: : → + +
The transformation of a proton to a neutron inside of a nucleus is also possible through electron capture: : + → + Positron capture by neutrons in nuclei that contain an excess of neutrons is also possible, but is hindered because positrons are repelled by the nucleus, and quickly annihilate when they encounter electrons.
When bound inside of a nucleus, the instability of a single neutron to beta decay is balanced against the instability that would be acquired by the nucleus as a whole if an additional proton were to participate in repulsive interactions with the other protons that are already present in the nucleus. As such, although free neutrons are unstable, bound neutrons are not necessarily so. The same reasoning explains why protons, which are stable in empty space, may transform into neutrons when bound inside of a nucleus.
The Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent electric dipole moment. The predicted value is, however, well below the current sensitivity of experiments. From several unsolved puzzles in particle physics, it is clear that the Standard Model is not the final and full description of all particles and their interactions. New theories going beyond the Standard Model generally lead to much larger predictions for the electric dipole moment of the neutron. Currently, there are at least four experiments trying to measure for the first time a finite neutron electric dipole moment, including: Cryogenic neutron EDM experiment being set up at the Institut Laue-Langevin nEDM experiment under construction at the new UCN source at the Paul Scherrer Institute nEDM experiment being envisaged at the Spallation Neutron Source nEDM experiment being built at the Institut Laue-Langevin
The antineutron is the antiparticle of the neutron. It was discovered by Bruce Cork in the year 1956, a year after the antiproton was discovered. CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles, so studying antineutrons yields provide stringent tests on CPT-symmetry. The fractional difference in the masses of the neutron and antineutron is . Since the difference is only about two standard deviations away from zero, this does not give any convincing evidence of CPT-violation.
The existence of stable clusters of 4 neutrons, or tetraneutrons, has been hypothesised by a team led by Francisco-Miguel Marqués at the CNRS Laboratory for Nuclear Physics based on observations of the disintegration of beryllium-14 nuclei. This is particularly interesting because current theory suggests that these clusters should not be stable.
The dineutron is another hypothetical particle.
At extremely high pressures and temperatures, nucleons and electrons are believed to collapse into bulk neutronic matter, called neutronium. This is presumed to happen in neutron stars.
The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly. Neutrons that elastically scatter off atoms can create an ionization track that is detectable, but the experiments are not as simple to carry out; other means for detecting neutrons, consisting of allowing them to interact with atomic nuclei, are more commonly used. The commonly used methods to detect neutrons can therefore be categorized according to the nuclear processes relied upon, mainly neutron capture or elastic scattering. A good discussion on neutron detection is found in chapter 14 of the book Radiation Detection and Measurement by Glenn F. Knoll (John Wiley & Sons, 1979).
Fast neutron detectors have the advantage of not requiring a moderator, and therefore being capable of measuring the neutron's energy, time of arrival, and in certain cases direction of incidence.
The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in neutron activation, inducing radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of nuclear reactors and nuclear weapons. The fissioning of elements like uranium-235 and plutonium-239 is caused by their absorption of neutrons.
Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.
A major use of neutrons is to excite delayed and prompt gamma rays from elements in materials. This forms the basis of neutron activation analysis (NAA) and prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei, particularly the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Because free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions). Free neutron beams are obtained from neutron sources by neutron transport. For access to intense neutron sources, researchers must go to a specialist neutron facility that operates a research reactor or a spallation source.
The neutron's lack of total electric charge makes it difficult to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. These methods have little effect on neutrons beyond a small effect of an inhomogeneous magnetic field because of the neutron's magnetic moment. Neutrons can be controlled by methods that include moderation, reflection and velocity selection.
Hydrogen-rich ordinary water affects neutron absorption in nuclear fission reactors: usually neutrons are so strongly absorbed by normal water that fuel-enrichment with fissionable isotope is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is therefore used in CANDU-type reactors, in order to slow (moderate) neutron velocity, to increase the probability of nuclear fission compared to neutron capture.
Cosmic radiation interacting with the Earth's atmosphere continuously generates neutrons that can be detected at the surface. Even stronger neutron radiation is produced at the surface of Mars where the atmosphere is thick enough to generate neutrons from cosmic ray spallation, but not thick enough to provide significant protection from the neutrons produced. These neutrons not only produce a Martian surface neutron radiation hazard from direct downward-going neutron radiation, but also a significant hazard from reflection of neutrons from the Martian surface, which will produce reflected neutron radiation penetrating upward into a Martian craft or habitat from the floor.
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture.
Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge. Fusion reactors which generate neutrons are likely to create around twice the amount of radioactive waste of a fission reactor, but the waste is composed of neutron-activated lighter isotopes, which have relatively short (50–100 years) decay periods as compared to typical half lives of 10,000 years for fission waste, which is long primarily due to the long half life of alpha-emitting transuranic actinides. Nuclear power#Solid waste
In many substances, thermal neutrons have a much larger effective cross-section than faster neutrons, and can therefore be absorbed more easily by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result.
Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly.
Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a neutron moderator. In reactors, typically heavy water, light water, or graphite are used to moderate neutrons.
D-T (deuterium-tritium) fusion is the fusion reaction that produces the most energetic neutrons, with 14.1 MeV of kinetic energy and traveling at 17% of the speed of light. D-T fusion is also the easiest fusion reaction to ignite, reaching near-peak rates even when the deuterium and tritium nuclei have only a thousandth as much kinetic energy as the 14.1 MeV that will be produced.
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-fissile heavy nuclei, and these high-energy fissions produce more neutrons on average than fissions by lower-energy neutrons. This makes D-T fusion neutron sources such as proposed tokamak power reactors useful for transmutation of transuranic waste. 14.1 MeV neutrons can also produce neutrons by knocking them loose from nuclei.
On the other hand, these very high energy neutrons are less likely to simply be captured without causing fission or spallation. For these reasons, nuclear weapon design extensively utilizes D-T fusion 14.1 MeV neutrons to cause more fission. Fusion neutrons are able to cause fission in ordinarily non-fissile materials, such as depleted uranium (uranium-238), and these materials have been used in the jackets of thermonuclear weapons. Fusion neutrons also can cause fission in substances that are unsuitable or difficult to make into primary fission bombs, such as reactor grade plutonium. This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain nuclear proliferation discussions and treaties.
Other fusion reactions produce much less energetic neutrons. D-D fusion produces a 2.45 MeV neutron and helium-3 half of the time, and produces tritium and a proton but no neutron the other half of the time. D-3He fusion produces no neutron.
Cross sections for both capture and fission reactions often have multiple resonance peaks at specific energies in the epithermal energy range. These are of less significance in a fast neutron reactor where most neutrons are absorbed before slowing down to this range, or in a well-moderated thermal reactor where epithermal neutrons mostly interact with moderator nuclei, not with either fissile or fertile actinide nuclides. However, in a partially moderated reactor with more interactions of epithermal neutrons with heavy metal nuclei, there are greater possibilities for transient changes in reactivity which might make reactor control more difficult.
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most nuclear fuels such as plutonium-239, making epithermal-spectrum reactors using these fuels less desirable, as captures not only waste the one neutron captured but also usually result in a nuclide which is not fissile with thermal or epithermal neutrons, though still fissionable with fast neutrons. The exception is uranium-233 of the thorium cycle which has good capture-fission ratios at all neutron energies.
This text is licensed under the Creative Commons CC-BY-SA License. This text was originally published on Wikipedia and was developed by the Wikipedia community.
