This is part of the Homeopathy journal club described here:
doi:10.1016/j.homp.2007.05.006
Copyright © 2007 Elsevier Ltd All rights reserved. The Memory of Water: an overview
Martin F. Chaplin
, a,

aDepartment of Applied Science, London South Bank University, Borough Road, London, SE1 0AA, UK
Received 10 May 2007; revised 23 May 2007. Available online 31 July 2007.Abstract
The ‘memory of water’ is a concept by which the properties of an aqueous preparation are held to depend on the previous history of the sample. Although associated with the mechanism of homeopathy, this association may mislead. There is strong evidence concerning many ways in which the mechanism of this ‘memory’ may come about. There are also mechanisms by which such solutions may possess effects on biological systems which substantially differ from plain water. This paper examines the evidence.
Keywords: memory of water; homeopathy; evidence-based medicine; water clusters
Article Outline
- Introduction
- The evidence
- Evidence against the ‘memory of water’
- What is ‘water’?
- Evidence for the ‘memory of water’
- Conclusions
- References
Introduction
The ‘memory of water’ is a snappy expression that has eased its way into popular language. The term is mostly associated with Jacques Benveniste following his and others’ allergy research work.1 These research teams showed that solutes subjected to sequential physical processing and dilution demonstrate biological effects different from those apparent using just the water employed for the dilutions. The ‘memory of water’ holds within its brevity of phrase the concept that much diluted solutions appear to behave as though they contain absent solutes that had once been present.
From that beginning, its use has grown to include whether the properties of water can show distinct properties over periods of time much longer than expected and dependent on the aqueous preparation’s previous history. Originally, the term was proposed within a homeopathic context for public dissemination in the popular media. It presented an idea that was easily appreciated by non-scientists, although at a simplistic level. This caught the public imagination and, at the time, proved to be great publicity for homeopathy. More recently, however, the term ‘memory of water’ has proven to be unnecessary baggage in the homeopathy debate. Proof, lack of proof, or simple disbelief that water has, or can have, a memory has quite unnecessarily been confused with proof over whether homeopathy may or may not be efficacious.
Editorial comment in the scientific press has subsequently drawn on whether water can indeed show any ‘memory’ of its prior history as direct ‘proof’ of whether homeopathy can be successful or not. Such linkage is quite unnecessary and may easily mislead as the two areas utilize fundamentally differing and entirely independent evidence and should therefore be considered separately. One, the other, both or neither of these phenomena may represent real effects; they are not interdependent. Linking the two today such that they both stand or fall together2 is as senseless as the non-sequitur of linking the efficacy of aspirin with its bitterness. There is no need to judge homeopathy by ascertaining whether water has a memory of past events any more than we should judge conventional medicine by our level of appreciation, or ignorance, of its detailed molecular action. Thus, whether homeopathy works or not is a mostly separate issue from the content of this paper and should be judged solely on the evidence presented copiously elsewhere. It follows that simply proving that water does have a memory does not prove that homeopathic medicines work. Other considerations have to be taken into account (see later) including how any specificity of action may arise in the therapeutic effect.
This paper concerns the memory of water: to what extent past events may influence the future behaviour of aqueous solutions. Although interpreted by some as also applying to the ‘memory’ of single molecules of water3 this is a red herring just as any discussion of the human memory in terms of the properties of a single molecule of ATP. Also misleading is to discuss it solely in terms of just H2O molecules as no such material as pure liquid H2O can exist; liquid water always contains other species such as H+ ions.
Here, I discuss the memory of water in terms of the real aqueous solutions that are likely to be more generally encountered as ‘water’, including those found in a homeopathic context. Of particular relevance is that the water used in homeopathic preparations, whether distilled or deionized or both or including ethanol, may still contain many and variable solutes including nanomolar to micromolar concentrations of ions.
The evidence
All scientific hypotheses should, of course, be examined in an unbiased manner with reliance on evidence rather than belief. However, agreement or disagreement with the concept that aqueous preparations may have a memory of past events arouses great emotion and has caused careers to flounder, quite independent of any unbiased examination of the evidence and its worth. Before any evidence is examined, the initial response of most scientists and non-scientists (including both the author and indeed Jacques Benveniste) is mostly one of deep scepticism. Indeed it is often stated, by people who do not believe in such a ‘memory of water’, that the burden of proof it requires should be much greater than for other scientific hypotheses.4 Such an attitude may itself be considered unscientific: the same level of supporting evidence should be accepted for all scientific developments. If a lower level of proof is set for hypotheses that fit prior beliefs then we bias our view of science in favour of such beliefs and may be easily misled. That such a process is generally used is self-evident and has resulted in the slow uptake of new ideas and the overly long retention of fallacious concepts.
The science surrounding the ‘memory of water’ is a confusion of evidence for an effect, data showing a lack of evidence for an effect, evidence and opinion that there should be no effect and lack of evidence that there is an absence of an effect; all of which may be obtained under different conditions with varying probabilities. Also, the observation of a phenomenon is usually accompanied by an explanation but the observation does not necessarily prove that the explanation is correct. Thus the explanation of his experimental observations by Jacques Benveniste as due to the ‘memory of water’ may or may not be correct, whereas the data that he published, and its correctness or otherwise, is quite independent of this explanation. Unfortunately, too often the explanation is examined more closely than the experimental data, which may lead to the data being rejected without due cause.
Evidence against the ‘memory of water’
Before describing the evidence for the memory of water, it is pertinent to discuss the evidence suggested by the many scientists who deny water its ‘memory’. Rather surprisingly, these do not concern the production or examination of experimental data showing no effect of their prior history on the properties of solutions. They mostly concern arguments involving the ease with which hydrogen bonds between water molecules may break. Individual hydrogen bonds do not last long in liquid water (about a picosecond). Based on this one fact the opinion may be proffered that the mesoscopic structure of water must change on about the same time scale.
Such arguments are completely fallacious as is easily recognized if metal hydrates or solid water (ice) are considered. In the case of ice the hydrogen bonds also only last for the briefest instant but a piece of ice sculpture can ‘remember’ its carving over extended periods. Cation hydrates exist and are commonly described with particular structure (eg the octahedral Na+(H2O)6 ion) but the individual water molecules making up such structures have but the briefest of residence times (<microseconds).
What such arguments fail to address is that the behaviour of a large population of water molecules may be retained even if that of individual molecules is constantly changing. Such behaviour is easy to observe: a sea wave may cross an ocean, remaining a wave and with dependence on its history, but its molecular content is continuously changing.
The remaining evidence presented against the memory of water concerns whether water clusters may retain their organization for time periods greater than a fraction of a second. Evidence denying the long-life of such water clusters is generally based on computer modelling but also includes NMR and diffraction data.5 There are several good reasons why such methods would not show any significant clustering properties for liquid water.
Computer simulations only operate for nanoseconds of simulated time, although taking hours or days of real time. Such short periods are insufficient to show longer temporal relationships, for example those produced by oscillating reactions.6 They also involve relatively few water molecules (of the order of 100–1000 or so) over small (nanometre) dimensions, insufficient for showing large scale (
micron) effects. They utilize models for the water molecules that are inherently flawed, showing poor correspondence to the real experimental properties of water (except for those properties on which they were individually based) and hence poor at predicting known properties and likely to be highly inaccurate at predicting unknown properties.7 NMR and diffraction both determine individual water molecules as structures averaged from throughout the sample (akin to averaging the world’s population of men and women and coming up with an illusory ‘average’ person) and are incapable of detecting imprecise and mobile clusters where components may change.
It is clear, however, that in the absence of other materials or surfaces (see below), the specific hydrogen bonding pattern surrounding a solute does not persist when the solute is removed. This may be demonstrated by the change in the water density as salt is removed. If there was ‘memory’ at work here, such water would retain the effective high density that existed in the presence of salt, but it does not. Also, no long-lived clusters made up of particular water molecules can be discerned (>1 ms, >5 μM) by NMR.8 On removal of hydrogen bonds when a hydrophilic solute is removed, the space vacated must be filled by aggressively hydrogen bonding water molecules. Such water would tend to alter the residual hydrogen bonding towards that pre-existing before the solute was originally added. Clearly such a process cannot be considered a memory effect involving ‘remembering’ the state before the solute was added.
Also, it is problematic to put forward a working hypothesis as to how small quantities of just H2O could have any different quantifiable effects when confronted with large amounts of complex and confounding solution in a human subject. Other materials must be present to stabilize such structuring against immediate destruction.
A further argument proffered against water having a memory involves drawing conclusions that water molecules must in their past been in multitudinous contact with almost infinite animate and inanimate objects and therefore cannot possibly ‘remember’ this whole history. I do not dispute this argument, but it is of no relevance to the state of known samples of liquid water, where the history concerns just the sample and is not the sum of the individual memories of all the molecules since the beginning of time (indeed individual H2O molecules only have lifetimes of fractions of a second).
Too often the final argument used against the memory of water concept is simply ‘I don’t believe it’. Such unscientific rhetoric is heard from otherwise sensible scientists, with a narrow view of the subject and without any examination or appreciation of the full body of evidence, and reflects badly on them.
What is ‘water’?
In order to properly discuss the memory of water it is first necessary to note what is meant by ‘water’ in this context. Here we assume that water is a solution of various, and varying, materials in liquid water. Real pure liquid water (if it could be created) would still consist of a number of molecular species including ortho and para water molecules, water molecules with different isotopic compositions such as HDO (D=deuterium) and
. Such water molecules occur as part of weakly-bound but partially-covalently linked9 molecular clusters containing one, two, three or four hydrogen bonds, and hydrogen ion and hydroxide ion species.
Apart from such molecules there are always adventitious and self-created solutes in liquid water. Distilled and deionized water still contain significant and varying quantities of contaminating ions. Often the criterion for ‘purity’ is the conductivity, but this will not show ionic contaminants at nanomolar, or even somewhat higher, concentrations due to the relatively high conductivity of the H+ and OH− ions naturally present. Other materials present will include previously dissolved solutes, dissolved gasses dependent on the laboratory atmosphere, gaseous nanobubbles, material dissolved or detached from the containing vessels, solid particles and aerosols (also dependent on the laboratory history) entering from the gas phase, and materials produced from water molecules and these other solutes on standing.
Liquid water is clearly a very complex system even before the further complexity of molecular clusters, gas–liquid and solid–liquid surfaces, reactions between these materials and the consequences of physical and electromagnetic processing are considered. It is remarkable that sceptics feel able to state with straight faces that they understand such systems to the extent that they know how they will behave in the absence of evidence on which to base their opinions. Certainly, there are plenty of scientific papers still being published which investigate the unpredictable behaviour of just parts of such complex solutions.
As applied to homeopathy, the memory of water concept should also be extended to the memory of aqueous ethanolic preparations. Addition of ethanol to water adds an important further dimension of complexity. Ethanol forms solutions in water that are far from ideal and very slow to equilibrate.10 Although usually considered a single phase, such solutions consist of a complex mixture dominated by water–water and ethanol–ethanol clusters, where hydrogen bonding is longer-lived than in water alone.11 They also favour nanobubble formation.12 Thus, the peculiar behaviour of aqueous solutions (as mostly discussed in this paper) is accentuated by the presence of ethanol.
Evidence for the ‘memory of water’
The concept of the memory of water revolves around whether the properties of such aqueous solutions change with time and/or processing and/or previous history. There are two aspects this problem. Can any memory of water effect be evidenced?, and is there satisfactory explanation for the appearance of memory in water? Clearly the first element should be sufficient. If there is evidence that the history of a sample of water affects its properties, then the ‘memory of water’ concept is proven without the need for a rationale for its action. However, it would seem that many scientists require an answer to the second part as well because the concept that water may possess a memory effect is perceived as so unlikely that simple proof that it happens is insufficient for them. In other areas of science experimental evidence is easily accepted where people ‘believe’ it to be true without a known rationale for its mechanism. An example is gravity. We believe it due to numerous observations but do not know how it exists. There is no requirement that the explanation for the memory of water is the correct explanation only that it must ‘seem’ reasonable. Of course, if it is also correct, that is a bonus!
There are several ways water can be shown to have a memory. As a simple example, human taste is quite capable of telling the difference between two glasses of water, processed in different ways (eg one fresh and one left undrunk for several days), where present analytical methods fail. There is a change, of course, but such a change would never be noticed by computer simulations on pure H2O. Vybíral and Voráček have shown that water changes its properties with time and its previous history.13 There is also a well-known ‘memory’ effect concerning the formation of clathrate hydrates from aqueous solutions whereby previously frozen clathrates within the solution, when subsequently melted, can predispose later to a more rapid clathrate formation.14 These examples may be explained, for example, by the presence of nanobubbles, extended chain silicates or induced clathrate initiators,15 respectively. Once an explanation is accepted, of course, the ‘memory of water’ seems no puzzle at all.
There are numerous other examples of the slow equilibration of aqueous solution. Thus, it can take several days for the effects of the addition of salts to water to finally stop oscillating16 and such solutions are still changing after several months showing a large-scale (
100 nm) domain structure.17 Also, water restructuring after infrared radiation persists for more than a day,18 and water photoluminescence changes over a period of days.19 Changes to the structure of water are reported to last for weeks following exposure to resonant RIC (resistance inductance capacitance) circuits.20 Conductivity oscillations (
0.5 Hz) at low concentrations of salts also show the weak tendency to equilibrium in such solutions.21
There is a strange occurrence, similar to the ‘memory of water’, in enzyme chemistry where an effectively non-existent material still has a major effect; enzymes prepared in buffers of known pH retain (remember) those specific pH-dependent kinetic properties even when the water is removed such that no hydrogen ions are present;22 these ions seemingly having an effect in their absence contrary to common sense at the simplistic level.
The effect of physical and electromagnetic processing is also evident from a number of studies; for example, due to changes in the amount of silica23 or redox molecules24 produced. Also manifest is that some ultra-dilute solutions, far beyond present detection by chemical analytical analysis, are known to have significant biological effects. A clear case of this is new-variant Creutzfeldt–Jakob disease, caused by infinitesimal amounts of prion protein.
There are several rational explanations as to how water may show different properties dependent on its previous history. In fact these are so obvious that it is a wonder why there is any further fuss about the ‘memory of water’. The current difficulty is choosing between the many reasonable explanations those that are the main causes for any memory effect. What is there in these solutions that depend on its history and which out of these constituents change so slowly as to still be showing effects at future times? To answer this question we need both thermodynamics and kinetics.
A water molecule in liquid water is never at a thermodynamic minimum for any appreciable (or measurable) length of time. This is because there are countless energy states in water with little difference in energy between them and the natural thermal fluctuations in liquid water are easily sufficient to allow change. Water consists of a heterogeneous mixture of states with little tendency for individual molecules to reside at a thermodynamic minimum; a process that is exacerbated by the presence of ethanol.
Solutions used in homeopathy contain many materials that may have biological effects. Of these, nanobubbles, nanoparticles and redox-active materials may separately or together cause biological responses. In terms of specificity, nanoparticles may present the most important possibility, if often overlooked. It is certainly true that such solutions show clear material differences from the diluting water used.25 The process of silica dissolution has been much studied26 ever since it was proven by Lavoisier over 200 years ago and fits with this argument. This may explain why glass is preferred over polypropylene tubes. A thorough investigation into the structural differences previously reported between homeopathically potentized (ie succussed and extremely diluted) and unpotentized nitric acid solutions showed that the effect was lost if different glassware was used.27
It should be noted that dissolved silica is capable of forming solid silicate particles with complementary structures (ie imprints) to dissolved solutes and macromolecules and such particles will ‘remember’ these complementary structures essentially forever.23 Redox-active material, such as superoxide anions and hydrogen peroxide,24 are associated with the control of many cellular processes and their presence is easily capable of giving rise to real biological responses. Nanobubbles are sub-micron scale gaseous bubbles that have long been subject of dispute as their existence does not fit with the commonly held belief in the Laplace equation relating the cavity internal pressures to the surface tension and cavity radius. However the evidence for nanobubbles is now overwhelming12 and the role of dissolved gas in water chemistry is likely to be more important than commonly realized;28 particularly involving the formation and development of these nanobubbles29 and the properties of their interfaces. Relevant to this are changes in carbon dioxide hydration, and hence pH, resulting from different hydrogen bonding effects. Nanoparticles may act by themselves or in combination with the nanobubbles to cause considerable ordering within the solution,30 thus indicating the possibility of solutions forming large-scale coherent domains.
A key feature of any difference between water before and after its use in preparing homeopathic dilutions is likely to be the shaking (succussion) between successive dilutions, and which may produce significantly increased concentrations of silicate, sodium and bicarbonate ions31 by dissolution of the glass tubes and from the atmosphere, respectively. Although not often recognized, except by microbiologists, such shaking can also produce aerosols saturating the laboratory atmosphere for extended periods and offering a route for the contamination of later dilutions.
Succussion involves the effect of pressure changes due to the shock waves produced. The magnitude of this pressure has not been well examined but may be estimated, from conservation of energy equating kinetic energy with strain energy, to be about 5–100 MPa dependent on the procedure. Equally increasing and decreasing (negative) pressure will be encountered so involving the compression and stretching of the hydrogen bonded network. Increasing pressure causes gas dissolution and decreasing pressure causes gas formation. Due to the slow kinetics of bubble initiation, it seems reasonable that such effects will mostly concern pre-existing gas nanobubbles in the bulk and at phase surfaces. Certainly bubbles could both grow and divide during such processing.
A further effect of pressure changes involves the silicate glass–liquid surface. Pressure waves would not only encourage dissolution but may dislodge nanoparticles of silicate (or other) material. It should be noted at this point that glass is not homogeneous but consists of nano-sized domains with differing structures.
Mechanically induced hydrogen bond breakage may also give rise to increased (but low) hydrogen peroxide formation24 and such effects have been reported to last for weeks,29 keeping the solution far from thermodynamic equilibrium.23 Such processes are well-known to produce long term oscillatory behaviour.[6] and [24] It may be relevant to note that the presence of hydrogen peroxide can take part in and catalyze further reactions with other reactive species such as molecular oxygen and dissolved ozone (not often recognized but also present in nanomolar amounts in ‘pure’ water) which may well vary with the number of succussion steps and their sequence and may offer an explanation for the changes in the effects of homeopathic preparations with the number of dilutions. Also of note are the known effects of low concentrations of reactive oxygen species on physiological processes such as the immune response.
That homeopathic preparations are always made using glass tubes may be indicative of the importance of silicates to these phenomena. If this is the case, there will be significant differences between using the same tube during dilution and using fresh glassware at each stage. If the former situation holds, as in the Korsakoff method, there may be marked consequences in terms of enduring changes to the glass surface and the continued presence or build up of materials at the surfaces, including the increased possibility of surface microbial contamination when ethanol is not used.
The processing of solutions also induces electric and electromagnetic effects, both of which seem to produce changes that have long lifetimes.32 The interface of solution with silica, for example, produces high localized fields (E
109 V m−1) caused by the partial charges on the atoms and the small distances between the surface and first hydration layer. Moreover, the flow of polar water molecules on succussion will itself create changes in electric field.
In addition to the breakage of hydrogen bonds, electromagnetic fields may perturb gas/liquid interfaces, produce reactive oxygen species33 and increase the differences in the properties between the ortho and para forms of water.34 Together with mechanical action, they will lower the dielectric constant of the water,35 due to the resultant partial or complete destruction of the hydrogen-bonded network.
Consequently, the solubility properties of the water will change during succussion and produce changes in the concentration of dissolved gases and hydrophobic molecules at interfaces thus encouraging their reaction (eg due to singlet oxygen, 1O2, or hydroxyl radical, OH·, formation) or phase changes (eg formation of extensive surface nanobubbles36).
These processes also result in the additional production of low concentrations of hydrogen peroxide and other redox materials24 with long-lasting effects. An interesting (and possibly related) memory of water phenomenon is the effect of water, previously exposed to weak electromagnetic signals, on the distinctive patterns and handedness of colonies of certain bacteria.37 Here, the water retains the effect for at least 20 min after exposure to the field.
In homeopathy as elsewhere, dilution is never perfect, particularly at low concentrations where surface absorption may well be a major factor, so that the real degree of dilution beyond the levels that can be analytically determined will always remain unproven. Residual material may be responsible for perceived differences between calculated and actual activity. Unless great care is taken, active material may also enter from the atmosphere even at the greatest dilutions. The water used for dilution is not pure relative to the putative concentration of the ‘active’ ingredient, with even the purest water considered grossly contaminated compared with the theoretical homeopathic dilution levels. This contamination may well have a major influence, and itself be influenced by the structuring in the water it encounters. Although it does, at first sight, seem unlikely that solutes in diluted ‘homeopathic’ water should be significantly different from a proper aqueous control, it has recently been cogently argued that the concentrations of impurities can change during the dilution process by reactions initiated by the original ‘active’ material38 and this process has been modelled to show how different potencies may give rise to differing effects.39
A further consideration about ‘the memory of water’ is that the popular understanding of how homeopathic preparations may work not only requires this memory but also requires that this memory be amplified during the dilution; this amplification, necessitated by the increase in apparent efficacy with dilution, being even harder to understand and explain. Samal and Geckeler have published an interesting, if controversial, paper40 concerning the effect of dilution on various molecules. They found that some molecules form larger clusters on dilution rather than the smaller clusters thermodynamically expected. Certainly, just the presence of one such large micron-sized particle in the ‘diluted’ solution could give rise to the noticed biological action. Of course some such preparations may be totally without action, not containing such clustered particles. However, this observation still sheds light on the phenomenon, which appears to disobey the second law of thermodynamics. A possible explanation is that some biologically-active molecules, such as the fullerene C60 involved in these experiments, can cooperatively form water networks to both surround and screen them. So long as such a network structure requires the help of more than one neighbouring such cluster to stabilize its formation then, in more concentrated solution, the molecules dissolve normally. However, as they are diluted no stabilizing clusters would be available close by if the solution was homogeneous. Consequently, the clusters would stabilize each other by coalescing to form larger clusters of biologically-active molecules within their own water network (ie they form their own aqueous phase). Overall the balance is expected to be rather fine between water cluster stabilization and particle precipitation and dependent on the particle’s ability to form a strongly bound hydration shell.
Although individual molecules of water cannot retain any memory of past hydrogen bonding for periods of more than a fraction of a second, the behaviour of water clusters can be entirely different (Figure 1), as shown previously for ice and cationic hydration. Water clusters are proven entities;41 their size and lifetime dependent on their physical and chemical environment. Liquid water is made up from a mixture of such clusters forming, changing and disappearing.
Display Full Size version of this image (30K) Figure 1. The lifetimes of clusters are independent of the lifetime of individual linkages. The figure is a two-dimensional representation of a three-dimensional phenomenon. The actual clusters of water molecules are not represented. Supposed that the star clusters (shown filled) may reform around key structures (shown as rhombuses labelled ‘r’, but closed ring oligomers of H2O in water). For each shifting cluster two units (filled circles) move to break up the existing cluster and help create a new cluster. The new clusters are identical to the old ones but only contain a proportion of the water molecules. Clusters may reform around any of the star arms.
The lifetime of a particular cluster containing specific water molecules will be not much longer than the life of individual hydrogen bonds (ie nanoseconds) but clusters can continue forever although with constant changing of their constituent water molecules. For example the icosahedral water cluster described by me42 contains one central-core structure but additionally 12 partly formed potential centre core structures on its periphery. Thus an icosahedral cluster can morph into a different but identical structure by shifting its centre; losing some molecules but gaining others. Although such complete icosahedral clusters are not thought important due to their likely low concentrations in ‘pure’ water under ambient conditions, under other conditions, with other solutes and phase interfaces and including related partial clustering such continuing structuring may well exist and be important.
This process may be considered similar to the existence of hydrogen ions in solution. There is no doubt that hydrogen ions are there, their concentration may easily be determined and they have uninterrupted and continuing effects, but individual hydrogen ions have only a fleeting existence (<nanosecond). The H+ is associated with a cluster of water molecules one moment, but in the next instant it disappears only to be replaced by a different H+ associated with an entirely different cluster of water molecules. Thus the hydrated hydrogen ion continues its existence but contains different atoms.
Water does store and transmit information concerning solutes, by means of its hydrogen-bonded network. Changes to this cluster network brought about by solutes may take some time to re-equilibrate. Succussion may also have an effect on the hydrogen bonded network (shear encouraging destructuring) and the gaseous solutes with critical effect on structuring43 and consequentially contribute to the altered heats of dilution with such materials.44
Recently, there has been some debate over ‘digital biology’; an idea originated by Jacques Benveniste that ‘specific molecular signals in the audio range’ (hypothetically the ‘beat’ frequencies of water’s infrared and far infrared vibrations) may be heard, collected, transmitted and amplified to similarly affect other water molecules at a receiver.45
This unlikely idea may be thought highly implausible but the evidence should be ignored at one’s peril. Note that as with the basic ‘memory of water’ concept, experimental confirmation of the phenomenon may not necessarily confirm the proposed mechanism.
Interestingly, however, electromagnetic emission has been detected during the freezing of supercooled water46 due to negative charging of the solid surface at the interface caused by surface ionization of water molecules followed by preferential loss of hydrogen ions. It is not unreasonable, therefore, that similar effects may occur during changes in the structuring of liquid water.
Finally, the ‘memory of water’ is considered by many to be the apparent physical result of a wider and complex holistic phenomenon.[47] and [48] Such a viewpoint lacks any mechanism for experimental testing at present.
Conclusions
There are a number of mechanisms for water to possess a ‘memory’. These have been described above and shown in the Table 1. The actual mechanism of action may differ between different ‘memory’ occurrences and may be the result of a combination of such phenomena. Some of these factors are clearly more likely, as described within this paper, and others can be easily eliminated or confirmed by closer examination of the procedures and/or analysis of the water.
Possible mechanisms by which water could achieve a ‘memory’
Specific mechanisms Non-specific mechanisms Remaining material on surfaces Silicates, dissolved and particular Aerosol material reintroduced Nanobubbles and their material surfaces Bacterial material introduced Redox molecules produced from water Imprinted silicates Natural water clustering Remaining particle clusters Stabilized water clustering Ions, including from glassware Ethanol solution complexity Note that, for homeopathy, ‘memory of water’ effects (if proven) not only require the solution to retain information on dilution but require this information to be amplified to negate the effect of the dilution. It is also of importance to note that non-specific mechanisms of action, such as activation of a non-specific immune response, may give rise to effects with specific health consequences. Much research work remains to be undertaken if these real and observable facts are to be completely understood.
1 E. Davenas, F. Beauvais and J. Amara et al., Human basophil degranulation triggered by very dilute antiserum against IgE, Nature 333 (1988), pp. 816–818. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
2 Wikipedia. Water memory.
http://en.wikipedia.org/wiki/Water_memory
, accessed on 28 April 2007.
3 M.L. Cowan, B.D. Bruner and N. Huse et al., Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O, Nature 434 (2005), pp. 199–202. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
4 J. Maddox, J. Randi and W.W. Stewart, ‘High dilution’ experiments a delusion, Nature 334 (1988), pp. 287–290.
5 J. Teixeira, Can water possibly have a memory? A sceptical view, Homeopathy 96 (2007), pp. 158–162. SummaryPlus | Full Text + Links | PDF (366 K)
6 I. Ferino and E. Rombi, Oscillating reactions, Catal Today 52 (1999), pp. 291–305. SummaryPlus | Full Text + Links | PDF (614 K) | View Record in Scopus | Cited By in Scopus
7 Chaplin M. Models for water.
http://www.lsbu.ac.uk/water/models.html
, accessed on 5 May 2007.
8 D.J. Anick, High sensitivity 1H-NMR spectroscopy of homeopathic remedies made in water, BMC Complement, Alternative Med 4 (2004), p. 15. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
9 Chaplin M. Hydrogen bonding in water.
http://www.lsbu.ac.uk/water/hbond.html#d
, accessed on 5 May 2007.
10 K. Takaizumi, A curious phenomenon in the freezing–thawing process of aqueous ethanol solution, J Solution Chem 34 (2005), pp. 597–612. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
11 C. Nieto-Draghi, R. Hargreaves and S.P. Bates, Structure and dynamics of water in aqueous methanol, J Phys Condens Matter 17 (2005), pp. S3265–S3272. View Record in Scopus | Cited By in Scopus
12 F. Jin, J. Ye, L. Hong, H. Lam and C. Wu, Slow relaxation mode in mixtures of water and organic molecules: supramolecular structures or nanobubbles?, J Phys Chem B 111 (2007), pp. 2255–2261. Full Text via CrossRef
13 B. Vybíral and P. Voráček, Long term structural effects in water: autothixotropy of water and its hysteresis, Homeopathy 96 (2007), pp. 183–188. SummaryPlus | Full Text + Links | PDF (279 K)
14 R. Ohmura, M. Ogawa, K. Yasuoka and Y.H. Mori, Statistical study of clathrate–hydrate nucleation in a water/hydrochlorofluorocarbon system: search for the nature of the “memory effect”, J Phys Chem B 107 (2003), pp. 5289–5293. Full Text via CrossRef
15 H. Zeng, L.D. Wilson, V.K. Walker and J.A. Ripmeester, Effect of antifreeze proteins on the nucleation, growth, and the memory effect during tetrahydrofuran clathrate hydrate formation, J Am Chem Soc 128 (2006), pp. 2844–2850. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
16 P.M. Wiggins, High and low-density water in gels, Prog Polymer Sci 20 (1995), pp. 1121–1163. SummaryPlus | Full Text + Links | PDF (3581 K) | View Record in Scopus | Cited By in Scopus
17 M. Sedlák, Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: II. Kinetics of the formation and long-time stability, J Phys Chem B 110 (2006), pp. 4339–4345. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
18 T. Yokono, S. Shimokawa, T. Mizuno, M. Yokono and T. Yokokawa, Clathrate-like ordering in liquid water induced by infrared irradiation, Jpn J Appl Phys 43 (2004), pp. L1436–L1438. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
19 V.I. Lobyshev, R.E. Shikhlinskaya and B.D. Ryzhikov, Experimental evidence for intrinsic luminescence of water, J Mol Liquids 82 (1999), pp. 73–81. Abstract | Abstract + References | PDF (531 K) | View Record in Scopus | Cited By in Scopus
20 C. Cardella, L. De Magistris, E. Florio and C.W. Smith, Permanent changes in the physico-chemical properties of water following exposure to resonant circuits, J Sci Explor 15 (2001), pp. 501–518. View Record in Scopus | Cited By in Scopus
21 S-Y. Lo and W. Li, Onsager’s formula, conductivity, and possible new phase transition, Mod Phys Lett B 13 (1999), pp. 885–893. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
22 A. Zaks and A.M. Klibanov, Enzymatic catalysis in nonaqueous solvents, J Biol Chem 263 (1988), pp. 3194–3201. View Record in Scopus | Cited By in Scopus
23 D.J. Anick and J.A. Ives, The silica hypothesis for homeopathy: physical chemistry, Homeopathy 96 (2007), pp. 189–195. SummaryPlus | Full Text + Links | PDF (242 K)
[24] V.L. Voeikov, The possible role of active oxygen in the Memory of Water, Homeopathy 96 (2007), pp. 196–201. SummaryPlus | Full Text + Links | PDF (136 K)
25 M.L. Rao, R. Roy, I.R. Bell and R. Hoover, The defining role of structure (including epitaxy) in the plausibility of homeopathy, Homeopathy 96 (2007), pp. 175–182. SummaryPlus | Full Text + Links | PDF (775 K)
26 J. Yang and E.G. Wang, Reaction of water on silica surfaces, Curr Opin Solid State Mater Sci 10 (2006), pp. 33–39. SummaryPlus | Full Text + Links | PDF (156 K) | View Record in Scopus | Cited By in Scopus
27 L.R. Milgrom, K.R. King, J. Lee and A.S. Pinkus, On the investigation of homeopathic potencies using low resolution NMR T2 relaxation times: an experimental and critical survey of the work of Roland Conte et al, Br Homeopath J 90 (2001), pp. 5–13. Abstract | PDF (150 K) | View Record in Scopus | Cited By in Scopus
28 R.M. Pashley, Effect of degassing on the formation and stability of surfactant-free emulsions and fine teflon dispersions, J Phys Chem B 107 (2003), pp. 1714–1720. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
29 L. Rey, Can low-temperature thermoluminescence cast some light on the nature of ultra-high dilutions?, Homeopathy 96 (2007), pp. 170–174. SummaryPlus | Full Text + Links | PDF (267 K)
30 Y. Katsir, L. Miller, Y. Aharonov and E. Ben-Jacob, The effect of rf-irradiation on electrochemical deposition and its stabilization by nanoparticle doping, J Am Electrochem Soc 154 (2007), pp. D249–D259. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
31 V. Elia and M. Niccoli, New physico-chemical properties of extremely diluted aqueous solutions, J Therm Anal Calorim 75 (2004), pp. 815–836. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
32 M. Yamashita, C.A. Duffield and W.A. Tiller, Direct current magnetic field and electromagnetic field effects on the pH and oxidation–reduction potential equilibration rates of water. 1. Purified water, Langmuir 19 (2003), pp. 6851–6856. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
33 M. Colic and D. Morse, The elusive mechanism of the magnetic ‘memory’ of water, Colloids Surfaces A: Physiochem Eng Asp 154 (1999), pp. 167–174. SummaryPlus | Full Text + Links | PDF (79 K) | View Record in Scopus | Cited By in Scopus
34 Andreev SN, Makarov VP, Tikhonov VI, Volkov AA. Ortho and para molecules of water in electric field. 2007; arXiv:physics/0703038v1 [physics.chem.-ph].
35 I. Danielewicz-Ferchmin and A.R. Ferchmin, Water at ions, biomolecules and charged surfaces, Phys Chem Liquids 42 (2004), pp. 1–36. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
36 P. Attard, Nanobubbles and the hydrophobic attraction, Adv Coll Interface Sci 104 (2003), pp. 75–91. SummaryPlus | Full Text + Links | PDF (448 K) | View Record in Scopus | Cited By in Scopus
37 E. Ben Jacob, Y. Aharonov and Y. Shapira, Bacteria harnessing complexity, Biofilms 1 (2004), pp. 239–263.
38 A. Morozov, Avogadro’s number and homeopathy, Homœopathic Links 16 (2003), pp. 97–100.
39 D.J. Anick, The octave potencies convention: a mathematical model of dilution and succussion, Homeopathy 96 (2007), pp. 202–208. SummaryPlus | Full Text + Links | PDF (187 K)
40 S. Samal and K.E. Geckeler, Unexpected solute aggregation in water on dilution, Chem Commun 21 (2001), pp. 2224–2225. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus
41 Chaplin M. The structure of liquid water: overview.
http://www.lsbu.ac.uk/water/abstrct.html
, accessed on 5 May 2007.
42 M.F. Chaplin, A proposal for the structuring of water, Biophys Chem 83 (2000), pp. 211–221. SummaryPlus | Full Text + Links | PDF (1850 K) | View Record in Scopus | Cited By in Scopus
43 A.V. Kondrachuk, V.V. Krasnoholovets, A.I. Ovcharenko and E.D. Chesnokov, Determination of the water structuring by the pulsed NMR method, Khim Fiz 12 (1993), pp. 1006–1010 (translated in Sov J Chem Phys 1994; 12: 1485–1492).
44 V. Elia, E. Napoli and R. Germano, The ‘Memory of Water’: an almost deciphered enigma. Dissipative structures in the extremely diluted aqueous solutions, Homeopathy 96 (2007), pp. 163–169. SummaryPlus | Full Text + Links | PDF (338 K)
45 Y. Thomas, The history of the Memory of Water, Homeopathy 96 (2007), pp. 151–157. SummaryPlus | Full Text + Links | PDF (400 K)
46 A.A. Shibkov, Y.I. Golovin, M.A. Zheltov, A.A. Korolev and A.A. Leonov, In situ monitoring of growth of ice from supercooled water by a new electromagnetic method, J Cryst Growth 236 (2002), pp. 434–440. SummaryPlus | Full Text + Links | PDF (192 K) | View Record in Scopus | Cited By in Scopus
47 L.R. Milgrom, Conspicuous by its absence: the Memory of Water, macro-entanglement, and the possibility of homeopathy, Homeopathy 96 (2007), pp. 209–219. SummaryPlus | Full Text + Links | PDF (736 K)
48 A.O. Weingärtner, The nature of the active ingredient in ultramolecular dilutions, Homeopathy 96 (2007), pp. 220–226.
Correspondence: Martin F Chaplin, Department of Applied Science, London South Bank University, Borough Road, London, SE1 0AA, UK.
Homeopathy
Volume 96, Issue 3, July 2007, Pages 143-150
The Memory of Water



 O. It is possible to measure accurately the typical time for which the three atoms are aligned (the lifetime of hydrogen bonds): it is of the order of 0.9 ps (9×10−13 s) at room temperature.
O. It is possible to measure accurately the typical time for which the three atoms are aligned (the lifetime of hydrogen bonds): it is of the order of 0.9 ps (9×10−13 s) at room temperature. (φu)crit.2. As the plate rotated further, a fourth equilibrium position point H, approximately identical with point D, was reached. From there, with decreasing angle φu, the position of the plate followed the previous section H–O and for φu=0° it returned to the original equilibrium position φd
(φu)crit.2. As the plate rotated further, a fourth equilibrium position point H, approximately identical with point D, was reached. From there, with decreasing angle φu, the position of the plate followed the previous section H–O and for φu=0° it returned to the original equilibrium position φd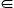 (0°, 360°, 0°), was equal to angle of torsion φu of the upper end of the filament, with accuracy of
(0°, 360°, 0°), was equal to angle of torsion φu of the upper end of the filament, with accuracy of  1 eV) and turn into a highly reactive singlet oxygen (O2(↑↓), also denoted, 1O2). A peculiar feature of 1O2 is that this electronically excited species may relax only to triplet state because oxygen, unlike other substances does not have ground singlet state. Since singlet–triplet transition is ‘forbidden’ by quantum physics laws, the lifetime of excited singlet oxygen is usually much longer than that of any other molecule in an excited singlet state. Probably that is why the reaction of singlet oxygen with water goes with sufficiently high probability—1O2 is long-living enough to find an appropriate catalytic environment for water oxidation.
1 eV) and turn into a highly reactive singlet oxygen (O2(↑↓), also denoted, 1O2). A peculiar feature of 1O2 is that this electronically excited species may relax only to triplet state because oxygen, unlike other substances does not have ground singlet state. Since singlet–triplet transition is ‘forbidden’ by quantum physics laws, the lifetime of excited singlet oxygen is usually much longer than that of any other molecule in an excited singlet state. Probably that is why the reaction of singlet oxygen with water goes with sufficiently high probability—1O2 is long-living enough to find an appropriate catalytic environment for water oxidation.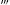 H→R
H→R H, then QS will be close to the maximum allowable concentration C, but if RS<H, there is no (positive) solution, and the concentration will die out to zero with repeated dilution–succussion cycles.
H, then QS will be close to the maximum allowable concentration C, but if RS<H, there is no (positive) solution, and the concentration will die out to zero with repeated dilution–succussion cycles.
